The Lorna Grindlay Moore, PhD Faculty Launch Fund
Due date for Letter of Intent: January 17, 2025
Funding Opportunities
The Lorna Grindlay Moore, PhD Faculty Launch Fund
The University of Colorado Denver (CU Denver) and University of Colorado Anschutz Medical Campus (CU Anschutz) proudly announce the next call for applications for the Lorna Grindlay Moore, PhD, Faculty Launch Award. Thanks to an endowment established by Dr. Moore, eligible Instructors and Assistant Professors are invited to apply for a one-year award of $40,000 to help launch their independent research programs. Preference will be given to those seeking to launch their independent research programs in women's health in Low- to Middle-Income Country (LMIC), but established faculty at the Associate or Professor rank who seek to extend their women's health-related research to LMICs are also invited to apply. The award can be expended over a two-year period. The awardee will be announced no later than May 26, 2025.
Due date for Letter of Intent extended to: January 17, 2025
Due date for Applications: March 21, 2025
Funds available as of July 1, 2025
Due date for Applications: March 21, 2025
Funds available as of July 1, 2025
About Lorna Moore, PhD
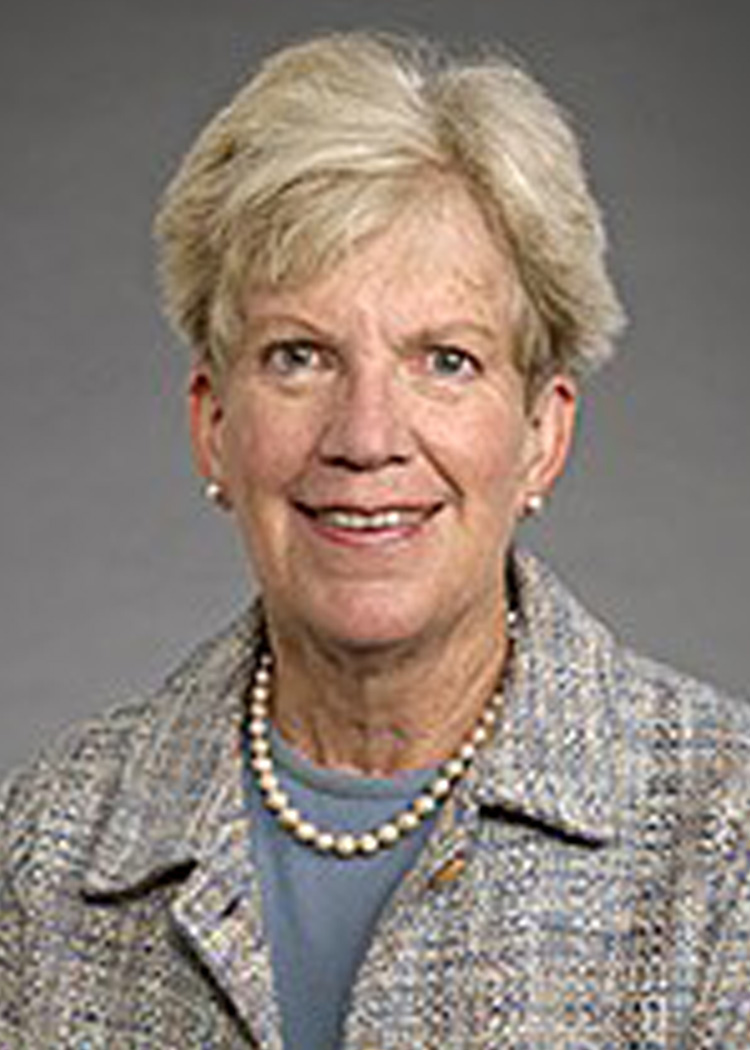
Lorna Grindlay Moore, PhD, is an internationally renowned researcher who has studied human adaptation to high altitude, having conducted studies in populations residing on three continents (North and South America, Asia). She has focused on the role of hypoxia in complications of pregnancy and fetal life, and the mechanisms by which evolutionary adaptation to environmental challenges has occurred. She obtained her BA in Anthropology from Smith College, pursued graduate studies in Cultural Anthropology at Brown University, and obtained her MA and PhD in Biological Anthropology at the University of Michigan. She joined the University of Colorado as a postdoctoral research fellow in the Cardiovascular Pulmonary Research Lab, rose through the ranks to become Professor and the founding chair of the CU Denver campus' Anthropology Department, while holding adjunct appointments in Medicine, Preventive Medicine, and Surgery/Emergency Medicine. She cofounded the CU Denver campus' PhD program in Health and Behavioral Sciences, the CU Anschutz Medical Campus' Center for Women's Health Research, and was a member of the steering committee for creating the Colorado School of Public Health. She then became Dean of the Graduate School of Arts and Sciences at Wake Forest University, before returning to the University of Colorado in 2012. Among Dr. Moore's many awards and honors, she is Past President of the Human Biology Association, Vice-President of the American Association of Physical Anthropology, a FASEB Outstanding Woman Scientist of the Year, a UC Denver Researcher of the Year, an Elizabeth Gee Mentorship Award recipient, and member of multiple national grant review panels (National Science Foundation and National Institutes of Health). She has authored over 200 peer-reviewed publications and been funded by the National Institutes of Health or other national organizations for more than 30 years.
Academic Enrichment Fund
The Academic Enrichment Fund (AEF) was established by the Department of Obstetrics and Gynecology in its "Cost Center Faculty Plan, 1989-1990" and is to be used to fund new and ongoing research programs and activities of the Department of Obstetrics and Gynecology. The AEF will provide funds to members of the department to support research endeavors. These funds will be disbursed only in response to specific written proposals, which shall be initially reviewed by the AEF Committee and will be subject to final approval by a majority vote of a quorum of the department faculty.
Purpose or Scope
The purpose of the AEF is to provide funding for research projects developed and led by faculty in the Department of Obstetrics and Gynecology to support career development. Priority is given to projects led by junior clinical faculty. Projects which are not led by junior clinical faculty may be considered, but a clinical faculty member must be a key investigator. Core laboratory equipment, supplies, or career development activities that are not related to a research project are outside the scope of the AEF.
Eligibility
All University of Colorado School of Medicine faculty, at the level of instructor or above, who are full-time faculty with a primary appointment in OB/Gyn and contribute clinical earnings to the AEF, are eligible to submit proposals. Projects may also consist of collaborations between clinical and basic science faculty, provided that the AEF-eligible, clinical co-investigator has a significant role and active participation in the project. Faculty carrying the Emeritus designation who are actively involved in the Department's teaching, research, or clinical activities are also eligible to apply for funding. Fellows are limited to one funded AEF grant proposal as Principal Investigator during their fellowship. Faculty will be limited to one AEF award as Principal Investigator at any given time and may not submit a second application within two calendar years of the start date (date of assignment of a speedtype) of a currently funded proposal.
Guidelines for Preparation of AEF Research Proposals
Format
Proposals for funding should be submitted in electronic format to the AEF Committee and include the following sections, where applicable. The minimum font size is 11 point with 0.5 inch margins. Section A, #s 1 through 5 may not exceed six pages, including all figures and tables. Applications that do not conform to these requirements will be returned without review.
- Description of Proposed Research
- Specific Aims and Hypotheses of the project.
- Background and significance.
- Innovation.
- Preliminary results, or summary of relevant studies.
- Research design and methods (including statistical analyses).
- Other Factors and Assurances: Please include human subjects protection and/or animal welfare, IRB, IACUC and other regulatory approvals. Also provide a statement of how this particular project will promote the career development of the investigator(s) and include the CV or biosketch of the Principal Investigator and mentor if applicable. If the Principal Investigator is not clinical faculty, discuss the specific role of the clinical faculty investigator.
- Literature Cited: Author, Title, Journal, Volume, Pages, Year
- Projected timeline*
- Appendices**
* AEF projects are typically funded for a maximum of 2 years. It is expected that all IRB approvals are to be completed within 1 year.
** Copies of relevant questionnaires, survey forms or letters of collaboration may be attached in an appendix.Applications must also include a “Lay Abstract” of up to one page, summarizing the specific aims, approaches and significance of the study in lay terms, understandable to a non-specialist. Should a grant be recommended by the AEF committee for funding, this abstract will be sent to all departmental faculty for review prior to their vote on the recommended proposal at a faculty meeting.
- Budget
A detailed line item budget must be included. The total amount requested must not exceed $12,000. If the AEF will provide only partial support for a project, please indicate the source of the remaining necessary funds. Examples of appropriate budgetary requests include participant stipends, research assistant time, equipment, reagents, animals and animal care, and data management and statistical support.
It is not anticipated that awards will be given to fund any full-time positions. Partial salary support may be requested reflecting the time commitment to the proposed project, however salary support must be clearly justified and priority will be given to funding of the project rather than personnel. Items ineligible for financial support include clerical or secretarial support and faculty travel expenses unless it is a direct research expense.
- Budget and Personnel Justification
The Budget and Personnel Justification should describe each item listed in the budget and provide the justification and information necessary to determine how the budget was calculated and the relationship between budgeted items and the project’s goals. The budget narrative must explain how each cost is necessary for the program, and match those items listed in the research proposal.
If requests are made for services or specific reagents (e.g. antibodies, pathology specimens, flow cytometry, real time PCR, gene arrays), indicate the number of samples to be processed and cost per sample or kit and provide documentation of these prices if possible. Requests for statistical or other support services should indicate hourly rate or per contract costs and justify the use of outside, non-institutional services or assays. For animal costs, indicate and justify the number of animals to be used and housing costs.
Please also provide a list of key personnel (Principal Investigator, Co-Investigators, collaborators, consultants, PRAs) and a brief (one paragraph) description of their specific role and percent effort on the project.
Review Criteria
Proposals will be evaluated based on the following criteria:
Significance: Project’s potential contribution to the body of knowledge and likelihood that research results will inform scientific concepts and methods.
Innovation: The extent to which the project employs novel concepts, approaches, or methods; challenges existing paradigms or develops new paradigms; or considers and existing problem from a new perspective.
Investigator(s): Investigator expertise and capacity to complete the proposed project or appropriate mentorship and/or collaboration plan. Priority is given to junior faculty, new investigators, and those with heavy clinical responsibilities.
Approach: The Hypotheses and Specific Aims should be clearly stated. What are the strengths and weaknesses of the methodology? Is the project feasible, are the methods sound and appropriate to assess the hypotheses and specific aims? Are the study design and statistical analyses clearly stated and appropriate for the project? Is the timeline adequate to complete the project as planned? Is the budget justified clearly and specifically and is supportive of the planned work?
Potential for future funding: Pilot research studies designed to provide preliminary data to support subsequent proposals for extramural funding or that may lead to new avenues of research are given priority.
Reviewers will assign a score for each criterion described above using the following scale:
| Overall Impact or Criterion Strength | Score | Descriptor |
|---|---|---|
| High | 1 | Exceptional |
| 2 | Outstanding | |
| 3 | Excellent | |
| Medium | 4 | Very Good |
| 5 | Good | |
| 6 | Satisfactory | |
| Low | 7 | Fair |
| 8 | Marginal | |
| 9 | Poor |
Scores will be averaged to determine the final score.
Review Process
Grant proposals will be reviewed by the AEF Committee typically in April and October. Requests for applications will be subject to availability of funds. The deadline for submission of grants will be approximately four weeks prior to each meeting and will be announced by e-mail. Should emergent funding be required for a project, the Committee can be convened for a special review at the request of the Department chair or a Division chief.
Any Committee member who is either a principal or co-investigator for a proposal or has a conflict of interest will disqualify him/herself from participating in the review. Any investigator making a proposal, whether a Committee member or not, may be asked to come to a Committee meeting to explain or clarify any aspect of the proposal or such clarification may be requested in writing.
The Committee will have the option of recommending acceptance of all or only part of the budgetary requests (i.e specific budget line items may be rejected or amended). Proposals not selected for funding may be resubmitted at the next cycle. Revised applications should include a one page response to reviewers’ comments. Depending on available funds, revised proposals may be eligible for expedited review by the Committee prior to the next submission deadline. Once the Committee's review process has been completed, its recommendations will be presented to the faculty for discussion and final decision by vote. Any faculty member may request a copy of AEF proposals that have been recommended for approval from the AEF Committee Chair or their designee.
While the number of awards granted per cycle will be dictated by the availability of funds, individual research grants will generally not exceed $12,000. The Committee may consider larger budget requests subject to a compelling and well–justified need. In addition, faculty may submit a competitive renewal of an existing AEF proposal to request additional funding. This renewal will be subject to full review by the AEF Committee and must include a progress report.
Administrative options of the Committee's review include recommendation for acceptance or rejection of the entire proposal as submitted or acceptance of portions of the proposal with recommendations to delete or modify certain aspects of the proposed study. All applicants will receive a written review of their proposal within one month of funding announcements.
Review Process
Once the proposal has been approved, the applicant will be notified via e-mail. Notices will contain the award number (assigned by the administrative assistant to the Committee), speedtype, title of the project, name of the principal investigator (Pl), award amount, and award duration. It is the responsibility of the Pl to administer the research project and budget as outlined in the proposals and to comply with all applicable COMIRB and IACUC regulations.
By accepting the award, the Pl agrees to the terms of the award which includes complying with the budget, providing an annual progress report, and completion of the study within three years. Failure to submit an annual report within 30 days of the anniversary of the award (speedtype start date) will result in suspension of funds. The annual progress report should include:
- A brief one page summary of progress.
- A budget report (including proposed budget and expenditures to date).
- A list or summary of abstracts or manuscripts that have been submitted, presented, or published as a result of the AEF project.
- Grant or other funding obtained to either support the AEF project or as a result of AEF funding.
- Changes to the personnel, specific aims, scope of project, project timeline, or budget.
If the project is not completed in three years, an extension may be granted but will be based upon the submission to the AEF Committee of a detailed progress report indicating reasons for delay in completion, as well as an estimated date of completion. AEF grants funding a project involving a Research Track Medical student will be completed in four years according to the expectations of that program. If any portion of the budgeted money remains unused by the completion of the project, this money will revert to the AEF for future awards.
At the completion of the study, the PI will be required to provide a report of findings to include a summary of data, conclusions drawn, initiatives undertaken to utilize the data obtained for additional research studies, possibilities for extramural funding, and a list of publications, abstracts, presentations, and grants related to the study. It is anticipated that such a report will be made available to the Committee by the grant deadline closest to the anniversary of the award. This report must also include an accurate accounting of funds awarded and disbursed. Failure to comply with the terms of this award will result in revocation of the award.
All expenditures related to the AEF program must be approved by the Department Administrator (or designee). A copy of all expenditures will be maintained by the Department Administrator or their designee as well as the Professional Research Assistant (or designee) for the PI.
CU REproduction (CURE) Grant Review Panel
The CURE Panel is a grant review panel consisting of 5 members from the University of Colorado Division of Reproductive Sciences, Department of OB-GYN, Section of Neonatology, Department of Pediatrics and a number of ad hoc members from the campus and across the US.
Members review grant proposals (any grant mechanism) to be submitted to major funding agencies (NIH, ADA, AHA, DOD, ACS, USDA, MOD etcetera) in reproduction and perinatology (broadly defined), submitted by investigators at the Department of OB-GYN and Division of Neonatology, Department of Pediatrics at CU Anschutz Medical Campus.
- Please ascertain that your submitted proposals comply with the instructions. Submissions will be pre-screened and if minimum requirements are not met, the proposal will be returned to the applicant.
- Each grant will be assessed by a minimum 3 scientific reviewers.
- Applicant will receive individual reviewers' comments and a Summary Statement. In contrast to the NIH Summary statement, reviewers will (if possible) suggest possible remedies for identified weaknesses.
Learn More about the CURE Panel
Review Cycles 2025:
| Review Cycle | Submission Deadline (5pm) | Panel Meeting | Critiques to Applicant |
|---|---|---|---|
| 1 | January 20 | ~ February 12 | ~ February 15 |
| 2 | April 8 | ~ May 1 | ~ May 2 |
| 3 | May 20 | ~ June 12 | ~ June 13 |
| 4 | August 5 | ~ August 28 | ~ August 29 |
| 5 | September 25 | ~ October 22 | ~ October 23 |
| 6 | December 2 | ~ December 19 | ~ December 20 |
Minimum Requirements for Submission:
- R-awards: Title, Project Summary, Specific Aims, Research Strategy, Literature Cited, Personnel/Budget justification, Biosketches for key personnel (for non-NIH applications: corresponding components).
- K-awards: Title, Project Summary, Specific Aims, Goals for Career Development and Research Strategy, Literature Cited, Personnel/Budget justification, Biosketches for key personnel including mentors (for non-NIH applications: corresponding components).
- The proposal has to be complete and nearly ready for submission to the funding agency. The purpose of the CURE Review Panel is not to replace critical input from mentors (junior applicants) and numerous revisions by the PI and the investigative team. Instead, the objective of the CURE Panel review is to take the application to the next level by identifying any weaknesses and potential flaws.
- Suggestions for ad-hoc, external (University of Colorado Anschutz Medical Campus, but not in OB-GYN or Neonatology or outside CU AMC) reviewers with particular expertise to review the application.
- Request for application/call for proposal details, if submitted in response to specific Program Announcement and Request for Proposals.
- For funding agencies other than NIH: Information on required format.
If resubmission: Summary statement/reviewers critique and Introduction/response to critique must be included.
Note that we use hard deadlines for submission, no exceptions.
Submit proposal as a single pdf file to Thomas Jansson, MD, PhD. For the purpose of scientific editing, also include the research proposal as a word file.
If you have questions or suggestions: Please contact Thomas Jansson, MD, PhD.
Submissions will be pre-screened and if minimum requirements are not met, the proposal will be returned to the applicant.
CU REproduction (CURE) Review Panel Roster 2024
Andrew Bradford, PhD
Division of Reproductive Sciences, Department of OB-GYN, University of Colorado School of Medicine
Thomas Jansson, MD, PhD
Division of Reproductive Sciences, Department of OB-GYN, University of Colorado School of Medicine
T. Rajendra Kumar, PhD
Division of Reproductive Sciences, Department of OB-GYN, University of Colorado School of Medicine
Hannah Dimmick, PhD
Division of Reproductive Sciences, Department of OB-GYN, University of Colorado School of Medicine
Theresa Powell, PhD
Divisions of Neonatology and Reproductive Sciences, Departments of Pediatrics and OB-GYN, University of Colorado School of Medicine
Grant Development Course
Each summer a grant writing course is offered to junior faculty and senior postdocs/clinical fellows. The format includes individual mentoring as well as a small group workshop, meeting once per week for six weeks with writing assignments to accomplish in between meetings.
Budget Development & Grant Submission Support
Email [email protected] for assistance in obtaining pricing and the development of your study budget.
The Resident Research Fund (RRF) was established by the Department of Obstetrics and Gynecology and is to be used to fund new and ongoing research programs and activities initiated by residents of the Department of Obstetrics and Gynecology. The RRF will provide funds to residents in the department to support research endeavors. These funds will be disbursed only in response to specific written proposals, which shall be initially reviewed by the RRF Committee and will be subject to final approval by co-committee members, senior faculty member, and department chair.
Purpose or Scope
The purpose of the RRF is to provide funding for research projects developed and led by current Ob-Gyn residents in the Department of Obstetrics and Gynecology at the University of Colorado to support and enhance the requirement to complete their scholarly activity. Priority is given to projects that are considered their primary resident research project (e.g. has received approval from the Assistant Program Directors of Resident Research). Core laboratory equipment, supplies, or travel activities that are not related to a research project are outside the scope of the RRF.
Eligibility
All University of Colorado School of Medicine Ob-Gyn residents are eligible to submit proposals in conjunction with their faculty mentor. Residents will be limited to one RRF award as Principal Investigator at any given time.
Guidelines for Preparation of RRF Research Proposals
Format
Proposals for funding should be submitted in electronic format to the RRF Committee and include the following sections, where applicable. The minimum font size is 11 point with 0.5 inch margins. Section A, #s 1 through 5 may not exceed six pages, including all figures and tables. Applications that do not conform to these requirements will be returned without review.
- Description of Proposed Research
Residents should submit their proposal in the following format:
- Specific Aims and Hypotheses of the project.
- Background and significance.
- Research design and methods (including statistical analyses).
- References.
- Projected timeline.*
- Appendicies.**
* RRF projects are typically funded for a maximum of 2 years. It is expected that all IRB approvals are to be completed within 1 year.
** Copies of relevant questionaires, survey forms or letters of collaboration may be attached in an appendix.Applications must also include a “Lay Abstract” of up to one page, summarizing the specific aims, approaches and significance of the study in lay terms, understandable to a non-specialist. Should a grant be recommended by the RRF committee for funding, this abstract will be sent to executive faculty for review prior to final vote on the recommended proposal by the RRF Committee.
- Budget
A detailed line item budget must be included. The total amount requested must not exceed $3,500. If the RRF will provide only partial support for a project, please indicate the source of the remaing necessary funds. Examples of appropriate budgetary requests include participant stipends, research assistant or medical student time, equipment, reagents, laboratory tests, and data management and statistical support.
It is not anticipated that awards will be given to fund any full-time positions. Partial salary support may be requested reflecting the time commitment to the proposed project, however salary support must be clearly justified and priority will be given to funding of the project rather than personnel. Items ineligible for financial support include clerical or secretarial support and travel expenses unless it is a direct research expense.
- Budget and Personnel Justification
The Budget and Personnel Justification should describe each item listed in the budget and provide the justification and information necessary to determine how the budget was calculated and the relationship between budgeted items and the project’s goals. The budget narrative must explain how each cost is necessary for the program, and match those items listed in the research proposal.
If requests are made for services or specific reagents (e.g. antibodies, pathology specimens, flow cytometry, real time PCR, gene arrays), indicate the number of samples to be processed and cost per sample or kit and provide documentation of these prices if possible. Requests for statistical or other support services should indicate hourly rate or per contract costs and justify the use of outside, non-institutional services or assays. For animal costs, indicate and justify the number of animals to be used and housing costs.
Please also provide a list of key personnel (Principal Investigator, faculty mentor, Co-Investigators, collaborators, consultants, PRAs) and a brief (one paragraph) description of their specific role and contribution to the project.
Review Criteria
Proposals will be evaluted based on the following criteria:
Significance: Project’s potential contribution to the body of knowledge and likelihood that research results will inform scientific concepts and methods.
Innovation: The extent to which the project employs novel concepts, approaches, or methods; challenges existing paradigms or develops new paradigms; or considers and existing problem from a new perspective.
Investigator(s): Investigator expertise and capacity to complete the proposed project or appropriate mentorship and/or collaboration plan. Priority is given to first year residents, although all other residents who have successfully completed one other research project will be eligible.
Approach: The Hypotheses and Specific Aims should be clearly stated. What are the strengths and weaknesses of the methodology? Is the project feasible, are the methods sound and appropriate to assess the hypotheses and specific aims? Are the study design and statistical analyses clearly stated and appropriate for the project? Is the timeline adequate to complete the project as planned? Is the budget justified clearly and specifically and is supportive of the planned work?
Potential for future funding: Pilot research studies designed to provide preliminary data to support subsequent proposals for extramural funding or that may lead to new avenues of research are given priority.
Reviewers will assign a score for each criterion described above using the following scale:
| Overall Impact or Criterion Strength | Score | Descriptor |
|---|---|---|
| High | 1 | Exceptional |
| 2 | Outstanding | |
| 3 | Excellent | |
| Medium | 4 | Very Good |
| 5 | Good | |
| 6 | Satisfactory | |
| Low | 7 | Fair |
| 8 | Marginal | |
| 9 | Poor |
Scores will be averaged to determine the final score.
Review Process
Grant proposals will be reviewed by the RRF Committee in May. Requests for applications will be subject to availability of funds. The deadline for submission of grants will be announced by e-mail.
Any Committee member who is a faculty mentor for a proposal or has a conflict of interest will disqualify him/herself from participating in the review. Any resident or faculty investigator participating in a proposal, whether a Committee member or not, may be asked to come to a Committee meeting to explain or clarify any aspect of the proposal or such clarifiaction may be requested in writing.
The Committee will have the option of recommending acceptance of all or only part of the budgetary requests (i.e specific budget line items may be rejected or amended). Proposals not selected for funding may be resubmitted at the next cycle. Once the Committee's review process has been completed, it’s recommendations will be presented to the executive faculty for discussion and final decision by vote.
While the number of awards granted per cycle will be dictated by the availability of funds, individual research grants will generally not exceed $3,500. The Committee may consider larger budget requests subject to a compelling and well–justified need.
Administrative options of the Committee's review include recommendation for acceptance or rejection of the entire proposal as submitted or acceptance of portions of the proposal with recommendations to delete or modify certain aspects of the proposed study. All applicants will receive a written review of their proposal within one month of funding announcements.
Administration of Award
Once the proposal has been approved, the applicant will be notified via e-mail. Notices will contain the award number (assigned by the administrative assistant to the Committee), speedtype, title of the project, name of the principal investigator (Pl), faculty mentor, award amount, and award duration. It is the responsibility of the Pl to administer the research project and budget as outlined in the proposals and to comply with all applicable COMIRB regulations.
By accepting the award, the Pl agrees to the terms of the award which includes complying with the budget, providing an annual progress report, and completion of the study within two years. Failure to submit an annual report within 30 days of the anniversary of the award (speedtype start date) will result in suspension of funds. The annual progress report should include:
- A brief one page summary of progress.
- A budget report (including proposed budget and expenditures to date).
- A list or summary of abstracts or manuscripts that have been submitted, presented, or published as a result of the RRF project.
- Grant or other funding obtained to either support the RRF project or as a result of RRF funding.
- Changes to the personnel, specific aims, scope of project, project timeline, or budget.
If the project is not completed in two years, an extension may be granted but will be based upon the submission to the RRF Committee of a detailed progress report indicating reasons for delay in completion, as well as an estimated date of completion. If any portion of the budgeted money remains unused by the completion of the project, this money will revert to the RRF for future awards.
At the completion of the study, the PI will be required to provide a report of findings to include a summary of data, conclusions drawn, initiatives undertaken to utilize the data obtained for additional research studies, and a list of publications, abstracts, presentations, and grants related to the study. It is anticipated that such a report will be made available to the Committee by the grant deadline closest to the anniversary of the award. This report must also include an accurate accounting of funds awarded and disbursed. Failure to comply with the terms of this award will result in revocation of the award.
All expenditures related to the RRF program must be approved by the Senior Faculty Oversight. A copy of all expenditures will be maintained by the Department Administrator or their designee.
Submission Deadline
All applications must be submitted to Resident Research Co-Directors by May 1st of the application year. Awards will be announced by June 1st of the application year.
Center for Women's Health Research (CWHR) Junior Faculty Research Development Awards (Seed Grants)
The Center's Junior Faculty Research Development Awards, or seed grants, jump-start ideas and help researchers build careers in women's health and sex and gender differences research. Our funding, mentoring, trainings, networking, and biostatistical support make it possible for junior researchers to obtain crucial pilot data and become independent investigators, effectively positioning them to secure future funding.
Learn MoreBuilding Interdisciplinary Research Careers in Women's Health (BIRCWH) Program
The Building Interdisciplinary Research Careers in Women's Health (BIRCWH) Program at the University of Colorado School of Medicine (UCSOM) provides career development to outstanding junior faculty members in key research specialties relevant to women’s health. The BIRCWH program’s aim is to provide bridging support to physician-scientists and scientists in the early stages of their professional careers so that they can achieve an independent research career. These junior faculty members are paired with senior investigators in a mentored, interdisciplinary scientific environment.
Learn MoreCCTSI Child and Maternal Health Pilot Grant (CMH-Pilot)
Provides one-year pilot awards to encourage cross-disciplinary and collaborative research focused on children of all ages as well as pregnant women and mothers of newborn and young infants that will ultimately improve child and maternal health.
Translational research is intentionally broadly defined, and includes any basic, pre-clinical or clinical research with promise to improve child health. Basic research at the cellular and molecular level, pre-clinical research in animal models, clinical research in humans, and outcomes or health services research are eligible if the ultimate goal is to improve human health.
RFA issued in July, Applications due in October, Selection in February.
Learn MoreWomen's Reproductive Health Research (WRHR) Career Development Program
at the University of Colorado
The WRHR Program was initiated in 1998 to provide the opportunity for obstetrician/gynecologists (OB-GYNs) who recently completed postgraduate clinical training to further their education and experience in basic, translational, and clinical research. Program sites provide departments of OB-GYN with an opportunity to create a talented pool of junior investigators with expertise in women's reproductive health research. The WRHR Program is sponsored by the NICHD, through its Gynecologic Health and Disease Branch (GHDB), and co-sponsored by the NIH Office of Research on Women's Health using the NIH Mentored Research Scientist Development Program Award (K12) mechanism.
The University of Colorado Denver is home to world class health care and research programs in women's health including reproductive disorders of adults and adolescents, perinatal/fetal biology, cancer biology, obesity, contraception, endocrinology, infectious disease and mental health disorders related to pregnancy. The goal of the UC-WRHR Center is to enable obstetricians and gynecologists to become successful physician scientists by providing outstanding research training and career development in these areas.
The CU WRHR Center has an outstanding group of mentors from basic science and clinical departments within the University of Colorado School of Medicine and from the Colorado School of Public Health to provide guidance for CU WRHR Scholars. Our mentors have an excellent record of training physician scientists in basic, clinical and translational research pursuits. CU WRHR Center Scholars have access to diverse education and research opportunities including masters and PhD programs offering specialized training in Reproductive Biology, Molecular Biology, Public Health and Clinical Science.
In 1999, the University of Colorado, Department of Obstetrics and Gynecology was awarded by NIH one of the first Women's Reproductive Health Research Career Development Centers in the United States. Funding for the CU WRHR Center has been renewed in 5-year funding periods from 2005-2025, with the upcoming 2025-2030 period pending.
The CU WRHR Center is located at the University of Colorado Anschutz Medical Campus in Aurora, Colorado, which is part of the greater Denver metro area. The campus integrates the Schools of Medicine, Pharmacy, and Dental Medicine; College of Nursing; Colorado School of Public Health; University of Colorado Hospital; Children's Hospital Colorado; and the new VA Hospital.
Eligibility Requirements
- MD or MD/PhD with completed residency in a US program (applicants with significant research experience will be given priority).
- No previous NIH independent research awards (R01, R03, or K award).
- United States citizen or permanent resident with an Alien Registration Card (Green Card).
Program Administration

Nanette F. Santoro, MD
Principal Investigator
Professor and E. Stewart Taylor Chair
Department of Obstetrics and Gynecology
Contact
Academic Office 1
12631 East 17th Avenue, MS B198-1
Aurora, CO 80045
Phone: (303) 724-2041
Fax: (303) 724-2061

T. Rajendra Kumar, PhD
Program Director
Professor and Edgar L., Patricia M. Makowski and Family Endowed Chair
Associate Vice Chair of Research
Department of Obstetrics and Gynecology
Contact
Research Complex 2
12700 East 19th Avenue, MS 8613
Aurora, CO 80045
Phone: (303) 724-8689
Fax: (303) 724-3512

Jeanelle Sheeder, MSPH, PhD
Program Director
Professor and Associate Vice Chair of Clinical Research
Departments of Obstetrics and Gynecology and Pediatrics
Contact
Academic Office 1
12631 East 17th Avenue, MS B198-2
Aurora, CO 80045
Phone: (303) 724-2272
Fax: (303) 724-2056

Nicole Goetz
Program Support
Division Administrative Assistant III
Contact
Research Complex 2
12700 East 19th Avenue, MS 8613
Aurora, CO 80045
Phone: (303) 724-3506
Fax: (303) 724-3512
Program Mentors
Anschutz Foundation Endowed Chair in Women's Integrated Mental and Physical Health Research at the Ludeman Center and Professor and Director for InterGenerational Stress and Health and the Director for Sex Differences Research in the Department of Psychiatry in the School of Medicine
Professor, Department of Family Medicine & Director of the Adult and Child Center for Outcomes Research and Delivery Science (ACCORDS) at the University of Colorado School of Medicine
Associate Professor, Divison of Reproductive Sciences, Department of Obstetrics and Gynecology
Levine-Kern Professor of Medicine and Immunology
Director, Mucosal Inflammation Program
Vice Chair for Research, Department of Medicine
Professor and Division Chief
William B. Goddard, MD, Endowed Professor in Urogynecology
Divisions of Urogynecology & Pelvic Reconstructive Surgery & Reproductive Sciences
Department of Obstetrics and Gynecology
Florence Crozier Cobb Endowed Professor and Chief, Division of Reproductive Sciences
Vice Chair of Research, Department of Obstetrics and Gynecology
Professor, Division Head & Scoville Endowed Chair, Division of Rheumatology, Department of Medicine
Professor and Edgar L., Patricia M. Makowski and Family Endowed Chair
Associate Vice Chair of Research
Divisions of Reproductive Sciences & Reproductive Endocrinology and Infertility
Department of Obstetrics and Gynecology
Professor, Division of Endocrinology, Metabolism and Diabetes, Department of Medicine
Emerging Mentors
Associate Professor
Divisions of Reproductive Sciences & Reproductive Endocrinology and Infertility
Department of Obstetrics and Gynecology
Current WRHR Scholars

Nancy Fang, MD, MS
(2023-Present)
Dr. Fang joined the Department of Obstetrics and Gynecology as a WRHR Scholar in August 2023. She completed her undergraduate degree in Chemistry and Global Health at Northwestern University. She worked for two years in a biology of addiction lab at Rockefeller University in New York City before moving to the University of Pittsburgh to complete her medical degree. She then moved to Denver to complete her ob-gyn residency at the University of Colorado School of Medicine. She completed her fellowship in Complex Family Planning at Columbia University Irving Medical Center and received a Master of Science in Epidemiology from the Mailman School of Public Health in New York City. Her research interests include equity in reproductive health and contraceptive improvement measures.

Nicole Marjon, MD, PhD
(2022-Present)
Dr. Marjon joined the Department of Obstetrics and Gynecology as a WRHR Scholar in 2022. She received her medical degree (MD) as well as her graduate degree (PhD) at the University of New Mexico. She then completed a residency in Obstetrics and Gynecology at Stanford University and Gynecologic Oncology Fellowship at the University of California San Francisco. Her current research is focused on understanding and modifying the immune microenvironment in ovarian cancer with the goal of developing novel therapeutics. She collaborates with established physicians and scientists at the University of Colorado and across the country, who share the same purpose of improving treatment options for patients with gynecologic malignancies.
Past WRHR Scholars
Aaron Lazorwitz, MD, MSCS
(2018-2023)
Dr. Lazorwitz an Associate Professor and Fellowship Director in the Division of Family Planning. He joined the Department of Obstetrics & Gynecology as a WRHR Scholar in June 2018. He received his MD from University of Texas Southwester Medical School and completed a fellowship in Family Planning at CU Anschutz in 2018.
Margo Harrison, MD, MPH
(2017-2022)
Dr. Harrison joined the Department of Obstetrics and Gynecology as a WRHR Scholar in June 2017. She received her MD from Albert Einstein College of Medicine in 2010 and completed fellowships in Family Planning and Global Maternal and Child Health at New York Presbyterian Hospital. Dr. Harrison's focus is on researching methods to improve diagnosis of labor dysfunction, transfer to a facility, and delivery of high-quality emergency obstetrical care in low-resource settings, while promoting access to comprehensive family planning services
Torri Metz, MD, MS
(2016-2018)
Dr. Metz is Associate Professor and Vice Chair of Research at University of Utah Health. She received her MD from the University of Colorado Denver in 2004 and also completed her residency at CU Anschutz in 2009. Dr. Metz earned an MS in Clinical Investigation and completed her fellowship in Maternal-Fetal Medicine at the University of Utah in 2012. Dr. Metz's research interests center on the use of marijuana in pregnancy and during lactation. She currently leads a research team that aims to better understand the prevalence of marijuana use in a state with legalized recreational marijuana.
Crystal Adams, MD
(2010-2015)
Dr. Adams received her MD from the University of Texas Health Science Center San Antonio in 2006. In 2010, she completed her residency in Obstetrics and Gynecology at the University of Texas Southwestern Medical Center – Austin. This was followed by a one-year fellowship in gynecologic and minimally invasive surgery at Virginia Mason Medical Center in Seattle. Dr. Adams has been a WRHR scholar and assistant professor at the University of Colorado since 2012. Her research interests are centered on the modulation of gene expression in ovarian malignancy, with a goal to better understand the mechanisms behind cancer cell resistance to chemotherapy and the development of molecularly targeted treatment modalities.
Camille Hoffman, MD
(2010-2015)
Dr. Hoffman received her medical degree from the Medical University of South Carolina in 2003, completed a residency in Obstetrics and Gynecology at University of Miami/Jackson Memorial Hospital in 2007, and completed a fellowship in Maternal Fetal Medicine at University of Colorado in 2010. Following fellowship, she became a faculty member and WRHR Scholar at the University of Colorado. Dr. Hoffman's research interests focus on the role of psychosocial and physiologic stress on pregnancy outcomes. She is evaluating ultrasound and noninvasive biomarkers of stress to assess maternal and child hypothalamic-pituitary-adrenal axis function.
Laxmi A. Kondapalli, MD, MSCE
(2011-2013)
Dr. Kondapalli received her medical degree from the University of Vermont College of Medicine in 2002 and completed her residency in Obstetrics and Gynecology at Northwestern University in 2006. She remained at Northwestern from 2006-2008 as a post-doctoral research fellow in reproductive biology lab of Teresa K. Woodruff, PhD. She completed her Reproductive Endocrinology and Infertility fellowship and received a Master of Science degree in Clinical Epidemiology at the University of Pennsylvania in 2011. Dr. Kondapalli is currently a staff physician at the Colorado Center for Reproductive Medicine and Director of the Colorado Fertility Preservation Program. She retains a Clinical Assistant Professor appointment at the University of Colorado Denver. Dr. Kondapalli's research interests focus on late effects of cancer therapy on gonadal function, fertility preservation methods, and childhood outcomes after assisted reproduction.
K. Joseph Hurt, MD, PhD
(2010-2012)
Dr. Hurt graduated with MD and PhD degrees from the Johns Hopkins School of Medicine. He completed his residency in Gynecology & Obstetrics also at Hopkins in 2009, then spent one year as a research fellow in the neuro-urology research laboratory of the Hopkins Brady Urological Institute. Dr. Hurt recently completed a fellowship in maternal fetal medicine at the University of Colorado Denver and is currently an Assistant Professor. His laboratory works to understand the role of the gastrotransmitters nitric oxide and hydrogen sulfide in regulating myometrial quiescence and parturition, with the goal of developing novel therapies to prevent preterm birth.
Irina Dimitrova, MD
(2005-2009)
Dr. Dimitrova received her MD from Sophia University, Bulgaria in 2001 and completed her residency in Obstetrics and Gynecology, Oakwood Medical Center, Dearborn, MI. She was accepted into the Gynecologic Oncology Fellowship at the University of Colorado. Her research interests focus on microRNAs in ovarian cancer and microarray analysis to identify mechanisms underlying the pathogenesis of uterine leiomyosarcomas and leiomyomata.
Monique A. Spillman, MD, PhD
(2006-2010)
Dr. Spillman received and MD/PhD from University of Texas, Southwestern in 1999 and completed her residency at Brigham and Women's Boston (2003). She was Gynecologic Oncology at Duke University from 2003 – 2006. Dr. Spillman was recruited CU Anschutz in 2006 as an Assistant Professor of Obstetrics and Gynecology and promoted to Associate Professor in 2012. In 2014, she was recruited to the Baylor Charles A. Sammons Cancer Center in Dallas Texas. She continues to be actively involved in gynecologic oncology research and clinical training. Dr. Spillman's research interests focus on understanding hormonal regulation of human ovarian cancer using mouse xenograft models.
Virginia D. Winn, MD, PhD
(2007-2010)
Dr. Winn received an MD, PhD, from the University of Rochester in 1996 and completed her residency in Obstetrics and Gynecology at the University of San Francisco in 2000. She was an MFM Fellow at UCSF from 2000-2003 and an Instructor/Adjunct Professor from 2003-2006. Dr. Winn was recruited to CU Anschutz as an Assistant Professor of Obstetrics and Gynecology with a joint appointment in Cell and Developmental Biology in 2006. She was promoted to Associate Professor in 2012. In 2014 she was recruited to Stanford University as an Associate Professor of Obstetrics and Gynecology and Director of the Division of Perinatal Biology. Dr. Winn's research interests are to understand the genes and molecular pathways modulated during the formation and function of the maternal-fetal interface. She is specifically interested in how these pathways contribute to obstetrical complications such as preeclampsia.
Lorraine Dugoff, MD
(2006-2008)
Dr. Dugoff received her MD from Georgetown University School of Medicine in 1989 and completed fellowships in Maternal Fetal Medicine and Clinical Genetics and the University of Colorado Health Sciences Center. She is currently is an Associate Professor, in the Department of Obstetrics and Gynecology, Perelman School of Medicine, University of Pennsylvania. Her research focuses on the identification of maternal serum markers for screening and prediction of preeclampsia, fetal chromosomal anomalies and perinatal birth defects.
Henry Galan, MD
(2000-2003)
Dr. Galan is Professor in the Department of Obstetrics and Gynecology, University of Colorado Denver. He is the Co-Director of the Colorado Fetal Care Center and has directed the Maternal Fetal Medicine Fellowship Program since 2000. Dr. Galan's research focuses on the hemodynamics of intrauterine growth restriction.
Radika Gogoi (nee Ghatge), MD, PhD
(2000-2005)
Dr. Gogoi is a Clinical Assistant Professor at Lankenau Institute for Medical Research and Lankenau Hospital, Wynnewood Pennsylvania. Following completion of her WRHR Fellowship she completed a fellowship in Gynecologic Oncology at New York University. Dr. Gogoi has been an attending clinician/researcher at the Siegfried and Janet Weis Research Center at Geisinger Medical Institute since 2011. Her research interests are HPV promoter for targeted cell death of HPV infected cervical cancer cells and the role of estrogens role in the development of pulmonary mycobacterium avium complex.