#18 in OB-GYN NIH Research Funding
$5,017,347 in NIH Funding in 2021
*Source: Blue Ridge Institute for Medical ResearchA tradition of producing effective, high-impact research that will improve the health of our population and future generations.
The history of research in the University of Colorado Anschutz School of Medicine Department of Obstetrics and Gynecology is rich and diverse. Seminal, historic achievements in Maternal-Fetal Medicine, Gynecologic Oncology and Reproductive Endocrinology have emanated from this institution, including but not limited to:
Our basic sciences investigators and clinical researchers are fully committed to extending this tradition of research excellence. To this end, we have established research programs in all of the major subdivision of OB-GYN and some non-traditional areas, such as mammary gland biology. We have developed full-spectrum bench-to-bedside research programs in most of these areas, and have been participants in numerous clinical trials, both NIH and industry-sponsored.
We have partnered with the community to engage our patients, through the Center for Women’s Health Research and through division-based community initiatives, and we have maintained old and created new collaborative research efforts that reach into geriatrics, metabolism and endocrinology, biochemistry, molecular biology, and –omics technology. In addition to these traditional scientific efforts, we collaborate intensely with each other, and self-evaluate on a regular basis to maintain the most effective research teams possible.
ARC is a full-service research support team serving all University of Colorado Anschutz School of Medicine Department of OB-GYN Faculty Researchers and Trainees. The team strives to provide expertise and resources with a high level of customer service.


PreAward Coordinator
Assists with Federal and Foundation proposal submissions; Works with principle investigators on post-submission requests including JITs and progress reports; Routes all incoming money with few exceptions.
Phone: (303) 724-0279

Regulatory and Finance Coordinator
Regulatory submissions; prepares invoices for industry sponsors; ensures compliance for clinical study subject compensation programs.
Phone: (303) 724-3859

Regulatory Manager
Oversees regulatory management for clinical research.
Phone: (303) 724-3859

Regulatory Coordinator
Assists with all the regulatory processes and submissions.
Phone: (303) 724-9765

Grants and Contracts Coordinator
Provides post award administration for federal and foundation grants and contracts; maintains financial projections and reconciliation of projects.
Phone: (303) 724-3053
This information is brought to you by the Department of OB-GYN Administrative Research Core (ARC). Please direct any questions to [email protected].

PA-25-422
(Parent F31)
Next Due: 4/8/2026

PA-25-423
(Parent F32)
Next Due: 4/8/2026

PA-25-301
(Parent R01 Clinical Trial Not Allowed)
Next Due: 6/5/2026 (New) or 7/5/2026 (Resubmission, Renewal, Revision)

PA-25-302
(Parent R03 Clinical Trial Not Allowed)
Next Due: 6/16/2026 (New) or 7/16/2026 (Resubmission, Renewal, Revision)

PA-25-304
(Parent R21 Clinical Trial Not Allowed)
Next Due: 6/16/2026 (New) or 7/16/2026 (Resubmission, Renewal, Revision)

PAR-25-110/PAR-25-111 (R01/R21)
Scope: translational research in maternal and pediatric pharmacology and therapeutics, encompassing tools, models, biomarkers, and other technologies
R01 Next Due: 6/5/2026 (New), 7/5/2026(Resubmission, Renewal, Revision)
R21 Next Due: 6/16/2026 (New), 7/16/2026 (Resubmission, Renewal, Revision)

PAR-25-159/PAR-25-158 (R34, R33/R61)
Scope: prevention of FASD or intervention for those affected by FASD throughout the lifespan
R34 Next Due: 6/16/2026 (New), 7/16/2026 (Resubmission, Renewal, Revision)
R33/R61 Next Due: 6/17/2026 (New), 7/17/2026 (Resubmission, Renewal, Revision)

PAR-25-185 (R01)
Scope: To develop new, innovative, and efficacious strategies for the molecular diagnosis, treatment, and prevention of human birth defects. Can use basic, applied, or database approaches.
Next Due: 6/5/2026 (New), 7/5/2026 (Resubmission, Renewal, Revision)

PAR-25-281/PAR-25-282 (R01/R21)
Scope: To advance mechanistic and translational research on the onset and worsening of mood and psychotic disorders during the menopausal transition or perimenopause.
R01 Next Due: 6/5/2026 (New), 7/5/2026 (Resubmission, Renewal, Revision)
R21 Next Due: 6/16/2026 (New), 7/16/2026 (Resubmission, Renewal, Revision)

Scope: To use existing and/or ongoing clinical research that rigorously examines outcomes in women, with specific attention to sex-specific biology of different exposures, interventions, diagnostics, and treatments.
Amount: $500,000 - $5,000,000
Deadline: Letters of interest accepted on a rolling basis

Scope: Invites researchers to suggest treatment guidelines to aid patient outcomes and satisfaction while generating data to drive best practices and obtain insight into drivers of contraceptive choice in treating gynecologic conditions.

Scope: Preliminary studies in new areas of research, with specific focus on in vitro fertilization, reproductive care for underrepresented populations, recurrent pregnancy loss, and precision medicine in clinical reproductive care.
Amount: up to $50,000 over 2 years
Deadline: April 20, 2026

Scope: Small, short-term studies on leading women’s health concerns, including cardiovascular disease, female cancers, the role of hormones in disease and stage-of-life health concerns, diseases disproportionately affecting women, and areas where sex differences need clarity.
Amount: up to $25,000
Deadline: March 14, 2026

Scope: Early career investigators interested in biomedical research to improve human health.
Amount: up to $250,000 over 1/2/3 years
Deadline: February 13, 2026
This information is brought to you by the Department of OB-GYN Administrative Research Core (ARC). Please direct any questions to [email protected].
Thanks for staying on top of it!
Thank you for keeping your document searchable!
Effective 10/28/2025 all new Human Subjects Research studies at CHCO must use Florence for documentation instead of paper binders (only the CHCO portion transfers). Current active studies are being backfilled into Florence by Friday, October 31st. There will be fees associated with industry-sponsored projects. Investigator-initiated studies and grant funded studies will be set up at no cost. You can contact [email protected] and [email protected] for further questions about this transition after Oct 31st.
The objective of the CURE Review Panel is to identify weaknesses and improve proposal quality. The next Submission Deadline is December 2nd at 5pm.
Minimum requirements for submission
Please include a Summary Statement and Response to Critique. Compile an almost complete proposal as a single PDF, include word file for scientific editing, and send it to [email protected]. For any questions, please contact Thomas Jansson.
For more information please visit: medschool.cuanschutz.edu/ob-gyn/research/funding-opportunities#ft-grant-development-1
NIH released NOT-OD-25-132, a new policy effective September 25, 2025 aimed at promoting fairness and originality in peer review.
Additionally, NIH has published a detailed FAQ addressing the use of generative AI in peer review and proposal development. All researchers and administrators are encouraged to review these guidelines to ensure compliance and uphold the integrity of NIH submissions.
In response to NIH NOT-OD-25-133, CU Anschutz and CU Denver have released updated guidance for NIH “Other Support” disclosures, aligning with federal requirements initially set to take effect October 1, 2025. The policy ensures transparency around all resources—financial and in-kind—that contribute to NIH-funded research and provides a training compliant with the updated policy.
We want to inform you that NIH’s Common electronic systems are currently experiencing issues with outgoing email communications. This affects notifications from ASSIST (including Human Subjects and Proposal updates), NIH Commons (including RPPRs), account creation confirmations, and proposal receipt acknowledgments.
Our campus announced adoption of new guidance and a rubric developed by the new School of Medicine Grant Alignment for Non-Federal Support (GRANT) committee to help investigators navigate recent NIH policies on research involving specific demographic groups and health disparities. This is a meaningful step toward aligning with evolving government requirements and supporting inclusive, impactful research for all our funders.
A study highlight was featured in CU Anschutz Today for fertility research between Irene Schauer, MD, PhD who studies insulin resistance and its impact on the cardiovascular system, and Nanette Santoro, MD, who studies infertility and obesity. Read about their research on insulin resistance and the impact of high-fat diets on fertility.
To align with new branding initiatives, please take a moment to update your email signature using the provided signature generator.
Additionally for new branding compliance, please use the updated letterhead template.
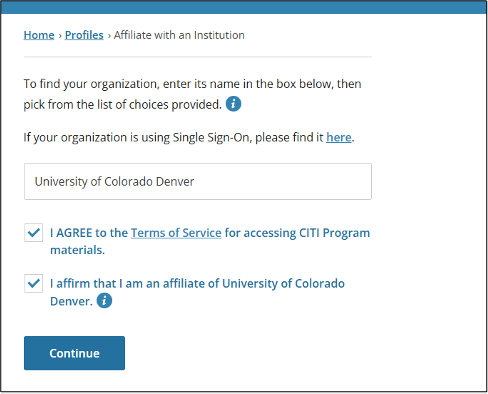
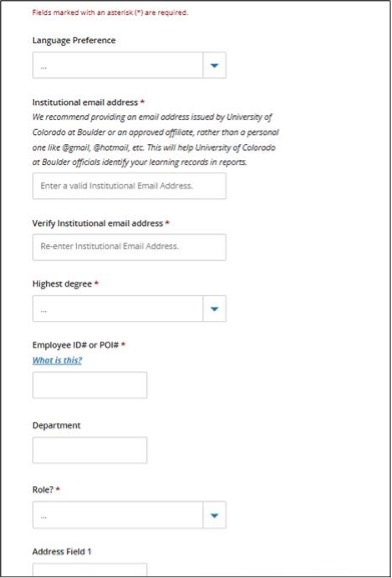
If your CITI training certificates list a previous institution, please add your affiliation to CU in the CITI Program website using these steps:

It is our pleasure to announce that the 2026 Lorna Grindlay Moore, PhD, Faculty Launch Fund award goes to Kelly Bogaert, MD! Dr. Bogaert is a clinical instructor at Denver Health and Assistant Professor of Obstetrics and Gynecology at the University of Colorado School of Medicine. Her research project is titled “A baseline assessment of the Surgical Trainer for InTerventions to Control Postpartum Hemorrhage (STITCH) in Rwandan Obstetrics and Gynecology Resident Physicians”. Congratulations to the awardee!
New guidance from the NIH requires that any NIHMS-funded research accepted for publication in a journal, on or after December 31, 2025, must be submitted to PMC upon acceptance for publication, for public availability without embargo upon the official date of publication. There are several ways to comply with these guidelines, as detailed on this page from the Strauss Library. In brief, publications containing information funded by the NIH can be published:
For any questions, please reach out to [email protected] at the Strauss Library to ensure you are complying with all funder requirements.
With new Marketplace upgrade, the process for submitting invoices to CU Medicine and UCHealth has changed as well as the Marketplace access role needed. Please see the guidance documents on how to submit payments on this new interface. You can reach out to [email protected] with any questions.
The next Conflict of Interest (COI) collection has begun as of Wednesday, August 6, 2025 and COI Disclosures will be due by the end of October 2025.
As a reminder, a COI Disclosure must be filed by faculty, officers, staff with fiscal roles, staff who negotiate on behalf of the university, and individuals who are engaged in research.
Questions? Click here to visit the COI webpage for more information on the University of Colorado Denver | Anschutz Medical Campus Conflict of Interest Policy or contact the COI staff at [email protected].
Have you received an email about your ePER? An ePER is an Electronic Personal Effort Report. Individuals who work on sponsored projects are required to certify their effort on those projects per Uniform Guidance. If this applies to you, you will receive emails after the end of each term (Spring, Summer, and Fall) asking you to complete the certification within 120 days. The subject line will read “Your ePER Requires Certification” and you will receive periodic reminder emails until the certification is complete. The instructions on how to find if you have outstanding ePERs and how to complete the process are (ATTACHED/LINKED) here. If you have any questions, please contact Pamela.[email protected].
NOT-OD-25-085: Effective January 1, 2025, the salary limitation for NIH is $225,700.
For active awards, including awards that have been issued in FY 2025 (continuation and new) that were restricted to Executive Level II, if adequate funds are available, and if the salary cap increase is consistent with the institutional base salary, recipients may rebudget funds to accommodate the current Executive Level II salary level.
The Department of Defense's Congressionally Directed Medical Research Program (CDMRP) has officially adopted the NIH Common Formats for the Biographical Sketch (biosketch) and Other Support documents.
What This Means for CDMRP Applicants
The objective of this course is to provide an opportunity for junior faculty, postdoctoral, and clinical fellows in the Department of OB-GYN to acquire knowledge and basic skills in grant writing.
Format: Individual mentoring + small group workshop, meets once a week for 5 weeks with writing assignments to accomplish in between meetings. Adequate time for writing (participant) and reading/providing feedback (mentor) must be available during this period.
Time: August 6 – September 17. Wednesdays 4:00-5:00 pm (in person). For more information, contact Thomas Jansson ([email protected])
The OB-GYN Manuscript Writing Course will take place from October 9-20th, hosted by Hannah Dimmick, department scientific editor. The course will be held virtually, 4-5pm each Wednesday during the time period (4 sessions total). Attendees are welcome to bring a manuscript to work on throughout the course (personalized feedback will be provided throughout if so), but it is not required. The course is geared towards fellows and junior faculty, but anyone is welcome to attend. The course will go over basics of scientific writing, as well as component-by-component instruction for developing an IMRaD-formatted paper, including methods, strategies, and best practices for writing. To register, please follow this link: https://ucdenverdata.formstack.com/forms/ob_gyn_manuscript_writing_course_registration
Just a quick reminder to help keep your clinical trials in top shape:
Make sure your FDA 1572 is up to date with the correct investigators and site addresses. Be sure to use the latest version dated April 8, 2025 for any updates going forward.
👉 Download the FDA 1572 here.
Each study team should maintain a current DOA log that lists all investigators, study coordinators, and research assistants involved in the trial.
👉 Download the DOA template here.
Don’t forget to keep an organized regulatory binder for each clinical trial. It should include:
👉 Download the template for Reg Binder here.
Keeping these documents current and accessible helps ensure compliance and smooth audits. Thanks for staying on top of it!
Access to OnCore will now be through the Advarra One platform.
If you experience any sign-in issues, please contact the OnCore team for assistance.
Stay tuned for updates and support during this transition!
To further support a successful transition to ScienCV and the Common Forms, NIH is postponing the May 25, 2025 implementation for all applications and Research Performance Progress Reports (RPPRs). NIH will issue future Guide Notices outlining the new effective date and additional implementation details as they are finalized.
NIH applicants and recipients must continue to use the current NIH Biosketch and Other Support format pages for applications, Just-in-Time (JIT) and RPPRs.
A revised W-9 form was released in 2024 and should be used moving forward for research study subject payments. As best practice, please visit www.irs.gov/FormW9 regularly to ensure that the form version you are using is the most recent. This will help you avoid delays in paying your study participants.
New research funding is set to start this summer from the Patient-Centered Outcomes Research Institute (PCORI) with Dr. Stephen Scott as co-lead with Dr. Sarah Brewer. Read about their research on the effectiveness of youth advisory boards.
 Charlie Graves is joining the Administrative Research Core as the Regulatory and Finance Coordinator and will work on regulatory submissions, preparing invoices for industry sponsors, and ensuring compliance for clinical study gift card programs. They are transitioning from their role as Senior Clinical Research Coordinator in the CU Division of Family Planning, where they have worked for six years. Before relocating from Pittsburgh to Denver in 2019, Charlie was a Research Assistant with the Center for Family Planning Research at the University of Pittsburgh Medical Center (UPMC). With over eight years of experience in OB-GYN clinical research, Charlie is now excited to pivot to the administrative side of OB-GYN research. In addition to their professional work, Charlie is pursuing a Master of Public Health in Population Mental Health and Wellbeing at the Colorado School of Public Health. Outside of work, they enjoy comedy, art, bird watching, and spending time with their partner and two cats.
Charlie Graves is joining the Administrative Research Core as the Regulatory and Finance Coordinator and will work on regulatory submissions, preparing invoices for industry sponsors, and ensuring compliance for clinical study gift card programs. They are transitioning from their role as Senior Clinical Research Coordinator in the CU Division of Family Planning, where they have worked for six years. Before relocating from Pittsburgh to Denver in 2019, Charlie was a Research Assistant with the Center for Family Planning Research at the University of Pittsburgh Medical Center (UPMC). With over eight years of experience in OB-GYN clinical research, Charlie is now excited to pivot to the administrative side of OB-GYN research. In addition to their professional work, Charlie is pursuing a Master of Public Health in Population Mental Health and Wellbeing at the Colorado School of Public Health. Outside of work, they enjoy comedy, art, bird watching, and spending time with their partner and two cats.
 Brenden Schatz is the Grants and Contracts Coordinator within the Administrative Research Core Team and will be providing post award administration for federal and foundation grants and contracts, maintaining financial projections, and reconciliation of projects. He is a recent graduate from the University of Wyoming with a bachelor's degree in finance and psychology. He is originally from Sheridan, Wyoming and has since moved to Denver to be around his family. In his free time, he researches urban equity and development in addition to reading and playing board games with friends and family.
Brenden Schatz is the Grants and Contracts Coordinator within the Administrative Research Core Team and will be providing post award administration for federal and foundation grants and contracts, maintaining financial projections, and reconciliation of projects. He is a recent graduate from the University of Wyoming with a bachelor's degree in finance and psychology. He is originally from Sheridan, Wyoming and has since moved to Denver to be around his family. In his free time, he researches urban equity and development in addition to reading and playing board games with friends and family.
With the Marketplace upgrade, there was a change in the process of submitting Medical Payment Vouchers. Your study team might have received a notice asking you to submit for Requestor access to be able to continue submitting these invoices for payment. The Procurement Service Center has also offered a custom fix for our department process flow to maintain Shopper-level access and obtain submission access for Medical Payment Vouchers. If you have not yet submitted for Requestor access, you can take the following trainings and send their certificate of completion (from Percipio/Skillsoft) to [email protected] to be granted access.
Two of these training courses should have already been taken by Shoppers related to their Shopper access. If you have any questions, please contact [email protected].
Please note that NIH will require the use of Science Experts Network Curriculum Vitae (SciENcv) to complete Common Forms and will require all Senior/Key Personnel to enter their ORCID ID into SciENcv in the Persistent Identifier (PID) section of the Common Forms for applications submitted on or after May 25, 2025. More information can be found NIH Notice NOT-OD-24-163:https://grants.nih.gov/grants/guide/notice-files/NOT-OD-24-163.html
Please ensure timely submission of your study results to ClinicalTrials.gov to maintain compliance with reporting requirements. Delays in reporting can result in significant penalties. For guidance and support, including assistance with results reporting, please visit this link. We also encourage you to review the FAQs for a detailed understanding of the requirements and potential consequences of delayed results. As the responsible party, it is crucial to stay informed and ensure compliance for your studies. Thank you for your attention to this important compliance matter.
Helpful reminders and tips for setting up and managing gift card programs:
"Did you know Health Data Compass offers Self-Service Data Delivery tools? These tools are the perfect option for those who want to explore our data assets and self-serve cohort data quickly and easily. Perfect for discovery and hypothesis generation. Our self-service databases provide a variety of deidentified patient information. Visit https://bit.ly/Compass_Data_Delivery to get started. Please note Self-Service Data Delivery will remain free to our customers after March 3, 2025, when Compass' Custom Data Delivery service pricing will be increasing. We want to assure our customers who have active projects that they will not be affected by this price increase. Visit https://bit.ly/Compass_Data_Delivery to learn more about our data delivery options."
Dean Sampson sent the following message in an email on February 24:
For the latest updates, FAQs, and guidance on federal actions affecting our operations, visit the CU Anschutz Federal Transition Update web page. You should also read the Federal Government Transition Update posted by the CU Office of Government Relations. Last Friday's update includes important information about matters that concern our campus community. Stay informed, stay connected, and trust that together, we'll continue advancing our academic, research, clinical, and public service missions.

Resources for Clinical Researchers including:

Resources for Laboratory Researchers including:

Resources for Laboratory Researchers including:

Resources for various internal and external research funding including:

Jayne Martin Carli, PhD, Post-doctoral Fellow in the McManaman-Monks Lab recently received an NIH K99/R00 Pathway to Independence Award on her continued study of human milk-derived cells to interrogate lactation insufficiency in the context of obesity and/or insulin resistance.

Theresa Powell, PhD is a professor in Pediatrics’ Neonatology Section as well as the Division of Reproductive Sciences in OB-GYN. She recently was awarded an NICHD R01 in July 2022 for 5 years. Title: Novel Roles for Phospholipids in Regulating Placental Function and in the Delivery of DHA to the Fetal Brain.
#18 in OB-GYN NIH Research Funding
$5,017,347 in NIH Funding in 2021
*Source: Blue Ridge Institute for Medical ResearchWRHR Award Recipients for over 20 years
The Department of OB-GYN has held a Women’s Reproductive Health Research Career Development Award since 1999.Forward Looking Research
The department focuses on cutting edge translational research with the objective to develop novel interventions in women’s reproductive health.


Check out all of our currently recruiting clinical studies in the CU Anschutz Research Studies database!


