
Hankinson Laboratory
Craniopharyngioma and Why Research is Needed
Adamantinomatous Craniopharyngioma (ACP) is a brain tumor that afflicts both children and adults. In children, it most commonly occurs between the ages of 5 and 14 years. It causes substantial injury to the brain, and also to structures that are critical for normal vision and hormone function. As a result of this type of damage and the our currently available treatments, ACP has been associated with the lowest Quality of Life scores of any pediatric brain tumor, and with significantly shortened lifespan relative to otherwise healthy people. 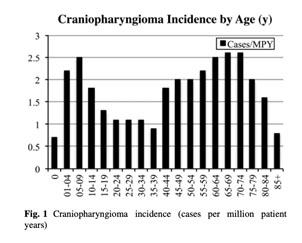 ACP is a complex tumor to study, partially because it has both solid and cystic (fluid) components. Further, the solid components include multiple cell types, which communicate with each other to cause tumor growth and brain injury. The details of this communication are not yet understood.
ACP is a complex tumor to study, partially because it has both solid and cystic (fluid) components. Further, the solid components include multiple cell types, which communicate with each other to cause tumor growth and brain injury. The details of this communication are not yet understood.
Interestingly, ACP always originates in an area at the base of the brain known as the sellar/suprasellar region. ACP hurts patients by damaging nearby structures such as the hypothalamus, nerves that mediate vision, and the pituitary gland. This leads to a debilitating group of symptoms that includes cognitive dysfunction, uncontrollable obesity, vision loss, sleep and memory impairment, and hormonal imbalances, among others. Our laboratory studies biological and clinical features of ACP with the goal of offering new treatments for patients and families afflicted by this tumor.
For many years, therapy for ACP has included surgery with or without radiation therapy (RT). There have also been efforts to instill medications directly into the fluid components of ACP. Unfortunately, these therapies are associated with the poor quality of outcomes that patients with ACP suffer. We need to do better. Currently, there are no medications that have been established to slow or stop ACP growth. Our lab is focused on identifying such therapies and bringing them into clinical use. 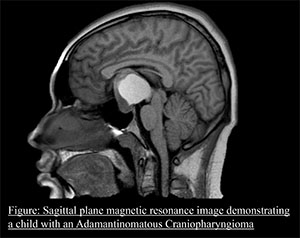 The Hankinson Lab has two primary research arms, each of which includes a high level of collaboration with colleagues on the Anschutz Medical Campus in Colorado and around the world. The first arm uses basic science methods to better understand the biology of ACP. This is highly dependent upon our collaborators from Advancing Treatment for Pediatric Craniopharyngioma, a multi-institutional North American group that shares tissue, clinical information and quality of life data regarding ACP (https://www.childrenscolorado.org/research-innovation/research-area/neurology-neurosurgery/atpc-consortium/). Using these resources, we have described gene expression characteristics of ACP and developed culture models of ACP tumor. We also collaborate closely with colleagues who have developed genetically engineered animal models and leverage other research tools to better understand ACP (https://www.ucl.ac.uk/child-health/research/developmental-biology-and-cancer/developmental-biology-birth-defects/pituitary-development). Building on data from our laboratory and others, there are now initial clinical trials of new therapies for ACP underway (https://connectconsortium.org/clinical-trials), (https://pnoc.us/clinical-trial/pnoc029/).
The Hankinson Lab has two primary research arms, each of which includes a high level of collaboration with colleagues on the Anschutz Medical Campus in Colorado and around the world. The first arm uses basic science methods to better understand the biology of ACP. This is highly dependent upon our collaborators from Advancing Treatment for Pediatric Craniopharyngioma, a multi-institutional North American group that shares tissue, clinical information and quality of life data regarding ACP (https://www.childrenscolorado.org/research-innovation/research-area/neurology-neurosurgery/atpc-consortium/). Using these resources, we have described gene expression characteristics of ACP and developed culture models of ACP tumor. We also collaborate closely with colleagues who have developed genetically engineered animal models and leverage other research tools to better understand ACP (https://www.ucl.ac.uk/child-health/research/developmental-biology-and-cancer/developmental-biology-birth-defects/pituitary-development). Building on data from our laboratory and others, there are now initial clinical trials of new therapies for ACP underway (https://connectconsortium.org/clinical-trials), (https://pnoc.us/clinical-trial/pnoc029/).
The second arm of research conducted in the Hankinson Lab harnesses computational methods (e.g. Artificial Intelligence and Machine Learning) to pioneer research platforms to improve our understanding of ACP. Using such tools, we have published and presented research that has improved our understanding of the genetic complexity of ACP, improved diagnostic accuracy using MRI and CT images, and identified predictable Quality of Life trajectories for ACP patients based on characteristics that are present at diagnosis. By continuing to apply advanced computational methods, and combine them with new biological insights, we aim to develop a highly comprehensive picture of ACP, which will facilitate the delivery of highly personalized and effective therapies against this uncommon but highly challenging disease.
Dr. Siddhartha Mitra, Assistant Professor of Pediatric Hematology/Oncology and Bone Marrow Transplant, co-leads our laboratory, bringing his expertise in immunology to advance the understanding of tumor microenvironments and disease progression. His research focuses on both adamantinomatous craniopharyngioma (ACP) and glioblastoma, with a strong emphasis on developing next-generation immunotherapies that harness the power of myeloid cells. His work explores the use of CAR-macrophages (CAR-Ms) and myeloid cell engagers (MCEs) as innovative therapeutic strategies to overcome the immunosuppressive tumor microenvironment. CAR-Ms are engineered to enhance tumor phagocytosis and stimulate anti-tumor immunity, while MCEs selectively recruit and activate myeloid cells against tumors. By integrating these cutting-edge approaches, Dr. Mitra’s lab aims to develop transformative treatments for glioblastoma and ACP, fostering collaboration across both disease areas to drive innovation in tumor immunology and therapy.