Optogenetic Interfacing with Nerves
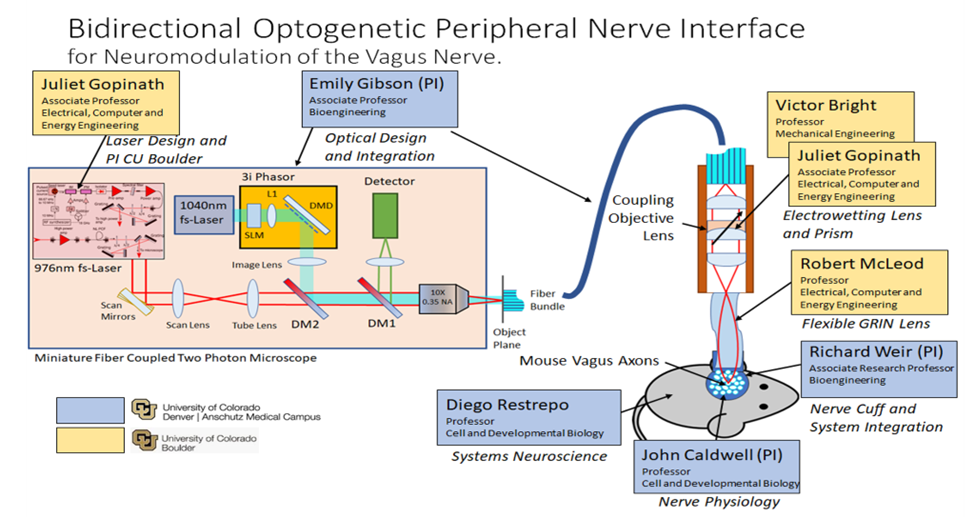
Figure 1: System diagram of our proposed optogenetic neural interface. We seek to develop a fiber coupled miniature two-photon microscope attached via a flexible GRIN lens to the vagus nerve of a live animal model. Our goal is an optical nerve interface that can record from and stimulate individual axons of the vagus nerve for closed loop optogenetic modulation of vagus nerve. With an optically based system we achieve: Axon level specificity; Organ level selectivity; Non-invasive interfacing.
Why talk to nerves using light?
To communicate with nerves, precision and non-invasiveness are key. Light can be focused precisely to both activate and read activity within a nerve with specificity not attainable with electrode-based approaches. These traditional electrode approaches usually deliver relatively broad and indiscriminate stimulation while also being invasive to the nerve. By using optical nerve cuff systems, we are working on non-tissue damaging chronic implantable devices to ‘talk’ to specific pathways within the nerve.
How can we use light to interact with nerves?
Novel Methods to ‘Talk’ to Peripheral Nerves - Our laboratory studies and develops novel techniques for interfacing with peripheral nerves, with the goal of stimulating and reading-out highly specific neural activity for rehabilitative and therapeutic purposes. We focus on optical (optogenetic) approaches which enable axon-level control of intervention with genetic targeting and spatially selective photostimulation. This research includes the engineering of implantable nerve devices, along with in vivo studies investigating the effect of targeted neural stimulation on organ function for disease therapies as well as prosthesis control. We are particularly interested in the therapeutic potential of the vagus nerve, which innervates the thoracic and abdominal organs. Photomodulation nerve cuff devices are in development to enable chronic studies in animal models for the investigation of systemic inflammation and post-traumatic stress disorder (PTSD) treatment. Recent applications also include the optical stimulation of pathways to modulate cardiac indices and pancreas endocrine function. To facilitate the interfacing of nerves with multi-photon microscopes we utilize an optical relay lens (GRIN-lens) incorporated nerve cuff.
3D-Printable Vagus Nerve Device for Chronic Photostimulation – This device will facilitate visible light illumination of the in vivo vagus nerve for optogenetic stimulation of select neural pathways. Current experiments include the targeting of cholinergic and glutamatergic pathways in the nerve to study how these neural pathways may be therapeutically beneficial in reducing systemic inflammation and treating PTSD.
3D Printing Optogenetic Interfaces
3D printers allow for the rapid iteration of different nerve cuff designs and allow for the customization of the nerve cuff for specific anatomies. Check out more about this project here.
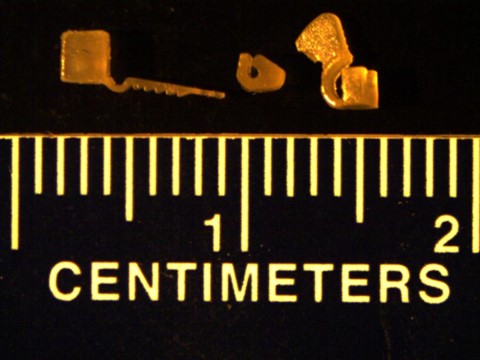
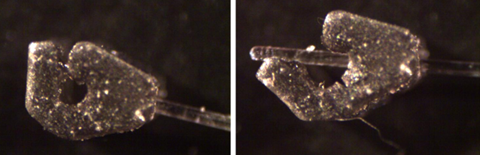
Figure 2: 3D Printed Neuromodulation Interfaces, or Nerve Cuffs, in PEGDA (Left: Self-Securing Optical Probe (Littich, 2020), Center: Passive Nerve Cuff, Right: Pull-Through Nerve Cuff)
Modulation of the Heart - Studies utilize transgenic rodent models as well as retrograde adeno-associated virus (AAV) delivered in the heart to specifically infect cardiac fibers of the upstream vagus nerve with light-sensitive opsin proteins. Stimulation of these vagal pathways, using both one-photon excitation and two-photon holographic excitation enable the perturbation of heart rate, ECG parameters and cardiorespiratory reflexes.1

Figure 3: Light-sensitive proteins were virally delivered to pathways in the vagus nerve and subsequently stimulated with light through a custom GRIN-lens integrated nerve cuff device leading to physiological changes including cardiac modulation.1
GRIN-Lens Nerve Cuff - A silicone pressure-molded nerve cuff was fabricated in 3D-printed maraging steel molds. The cuff incorporated a ratcheted strap mechanism to fix the nerve within the device. A GRIN lens provides an optical relay to interface the nerve with microscopes and other laser sources.

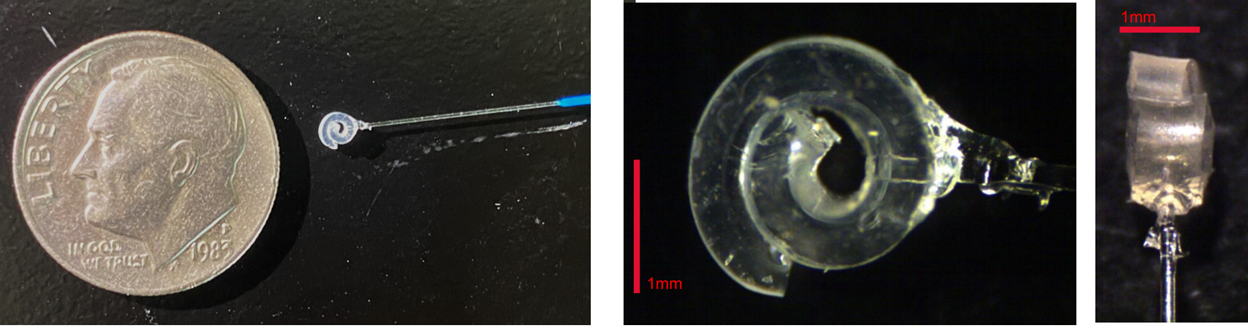
Figure 4: (A) 3D-Printed maraging steel molds for device fabrication. Progress made in nerve cuff manufacturing. (B) Diagram of the finished cuff in situ on the vagus nerve and next to a penny for scale. (C) Imaging of fluorescently labeled axons in the vagus nerve in vivo. After 83 days, it is still possible to image axons through the implanted GRIN.
Modulation of the Pancreas - We study whether optical activation of defined nerve pathways to the pancreas stimulates therapeutically beneficial effects on insulin and glycemic control. Our studies have demonstrated the stimulation of insulin secretion along with reduction in blood glucose levels by targeting cholinergic pathways2 – an effect not observed with electrical vagus nerve stimulation.

Figure 5:Optogenetic photostimulation of cholinergic fibers in the pancreas (top) and in the cervical vagus nerve (bottom) may be effective in reducing blood sugar by stimulating insulin secretion.2
Optical Measurement of Neural Activity In Vivo:

Figure 6: In Vivo GCaMP6s transients from vagus nerve axons in ChAT-GCaMP6s mice. (a) Two-photon optical section within the vagus nerve and activity-dependent calcium transients recorded in three axons (ROI1, ROI2, ROI3) in response to a 2-second electrical stimulation of 50 Hz and 70 Hz (black bar). A graded response to stimulus frequency is observed in two of the three responsive axons. (b) Activity-dependent calcium transient recorded in a separate mouse in response to a 10-action potential burst (mean of 3 axonal responses). (c-e) Spontaneous calcium transients recorded with no stimulation present.3
Selective Photostimulation with Spatial Light Modulation:

Figure 7: In Vivo GCaMP6s transients from vagus nerve axons in ChAT-GCaMP6s mice. (a) Two-photon optical section within the vagus nerve and activity-dependent calcium transients recorded in three axons (ROI1, ROI2, ROI3) in response to a 2-second electrical stimulation of 50 Hz and 70 Hz (black bar). A graded response to stimulus frequency is observed in two of the three responsive axons. (b) Activity-dependent calcium transient recorded in a separate mouse in response to a 10-action potential burst (mean of 3 axonal responses). (c-e) Spontaneous calcium transients recorded with no stimulation present.3
References
- Fontaine, A. K., Futia, G. L., Rajendran, P. S., Littich, S. F., Mizoguchi, N., Shivkumar, K., Ardell, J. L., Restrepo, D., Caldwell, J. H., Gibson, E. A. & Weir, R. F. Optical vagus nerve modulation of heart and respiration via heart ‑ injected retrograde AAV. Sci. Rep. 1–12 (2021). doi:10.1038/s41598-021-83280-3
- Fontaine, A. K., Ramirez, D. G., Littich, S. F., Piscopio, R. A., Kravets, V., Schleicher, W. E., Mizoguchi, N., Caldwell, J. H., Weir, R. F. & Benninger, R. K. P. Optogenetic stimulation of cholinergic fibers for the modulation of insulin and glycemia. Sci. Rep. 1–9 (2021). doi:10.1038/s41598-021-83361-3
- Futia, G. L., Fontaine, A., Littich, S., McCullough, C., Restrepo, D., Weir, R., Caldwell, J. & Gibson, E. A. In vivo holographic photo-stimulation and two photon GCaMP6 imaging of vagus nerve axons using a GRIN lens integrated nerve cuff. in Proceedings of SPIE: Optogenetics and Optical Manipulation 2019 28 (2019). doi:10.1117/12.2521830