Spiral Nerve Cuff for Systemic Inflammation
Vagus nerve stimulation (VNS) is a therapeutic technique clinically approved to treat depression, stroke recovery, migraines, and epilepsy. It is also being studied in the treatment of inflammatory diseases, such as Crohn’s and rheumatoid arthritis, as VNS has been shown to have anti-inflammatory effects.1 VNS reduces the inflammatory response by acting through the cholinergic anti-inflammatory pathway to attenuate production of pro-inflammatory cytokines, such as TNF-⍺ and IL-6.2,3 Current VNS methods utilize non-specific, electrode-based systems which can activate off-target pathways and lead to complications. Optogenetic methods can stimulate cells in a highly controlled manner with specific targeting.
How do we target the nerves?
Utilizing optogenetic techniques, a spiral, silicone nerve cuff with an embedded µLED, like shown in Figure 1, is being developed. Our device capitalizes on these optogenetic methods, applying them to VNS to provide specific, targeted stimulation of the vagus nerve for the study and regulation of VNS-mediated inflammatory diseases. The cuff is designed to “self-size” to the nerve, negate the need for sutures for securement, minimize nerve damage, and reduce off-target complications through optical stimulation.
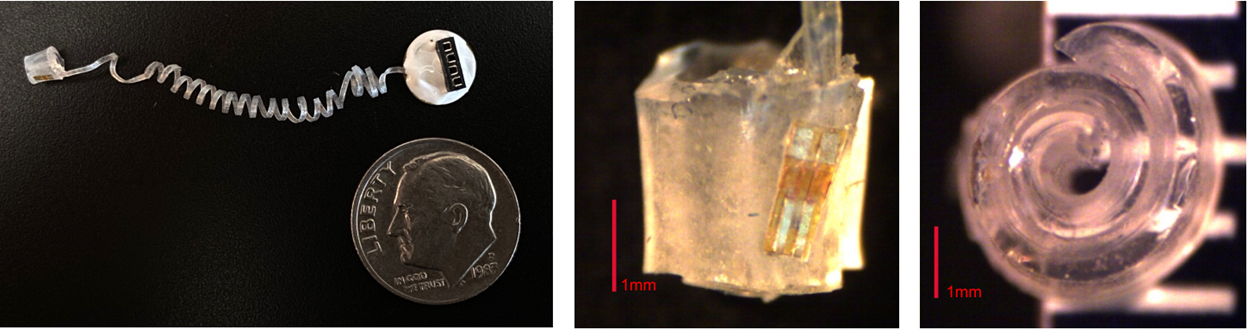
Figure 1: Nerve Cuff 1.0 - created using MDX4-4210 silicone. The nerve cuff was embedded with a 473 nm μLED.
Nerve Cuff 1.0 was implanted on the cervical vagus nerve of a ChAT-Cre x ChR2 mouse. The nerve was stimulated with the embedded μLED: 5Hz, 10% duty cycle. As shown in Figure 2, when implanted, the device successfully stimulates the vagus
nerve via optical stimulation, mirroring the effects of electrical VNS.
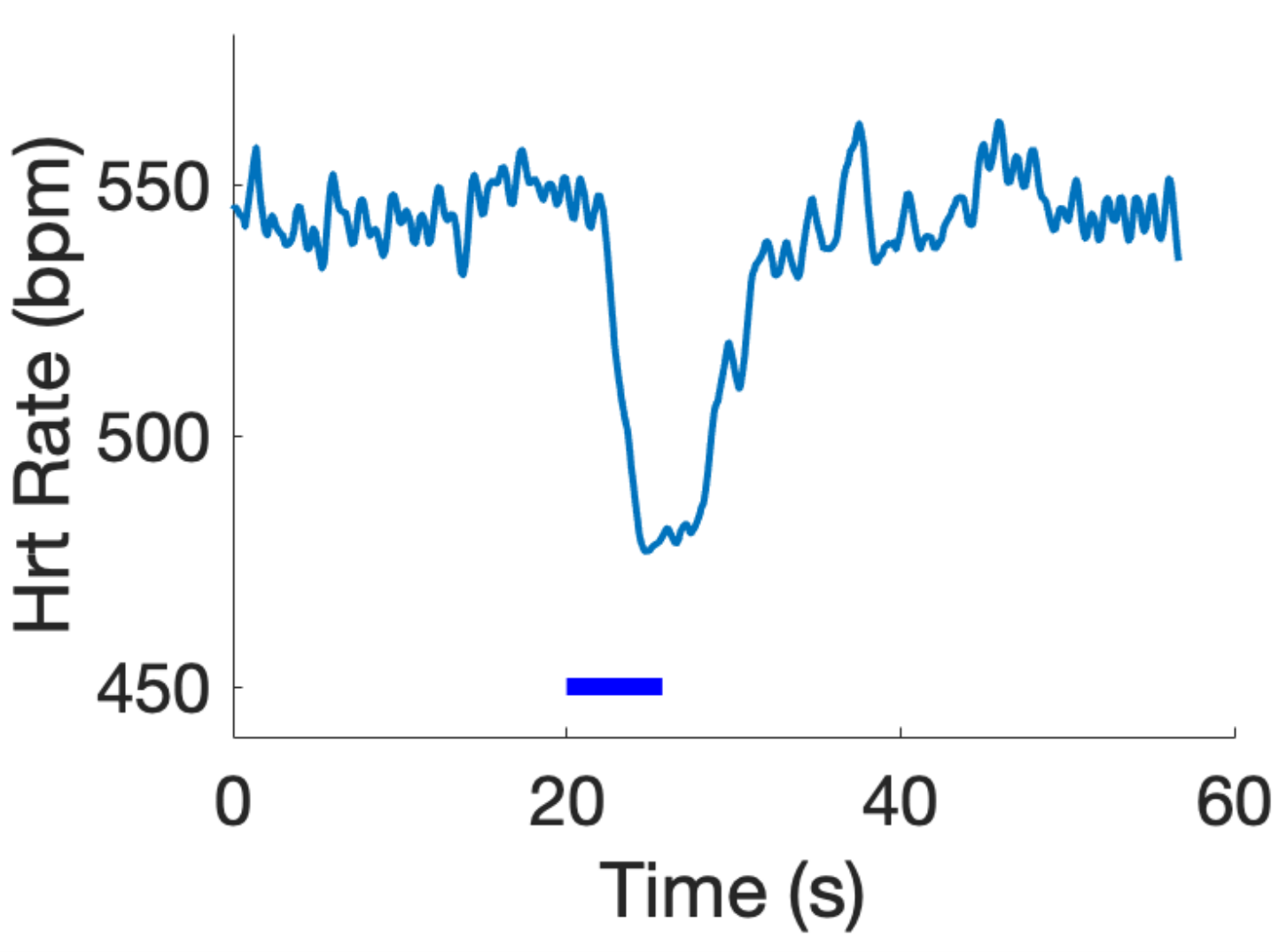
Figure 2: Example heart rate modulation in response to optical stimulation (blue bar), mirroring the effects of electrical VNS and validating the optical stimulation with the spiral nerve cuff.
Nerve Cuff 1.0 was iterated and redesigned to give Nerve Cuff 2.0 which was used in the pilot experiments for this study, shown in Figure 3. Due to supply chain issues, the μLED was replaced with an optical fiber for the optical stimulation. This allowed for validation of the optical fiber with this cuff as well.
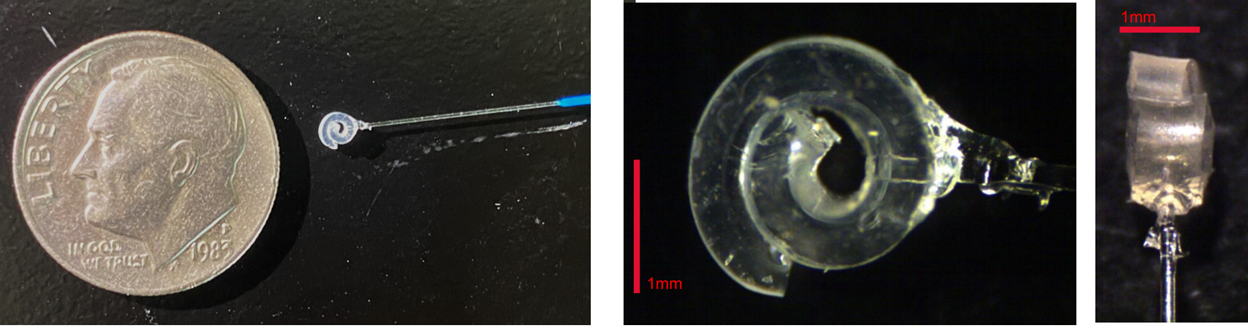
Figure 3: Nerve Cuff 2.0 - created with MDX4-4210 silicone. Embedded with a ThorLabs FP200URT Optical Fiber instead of a μLED.
How do we reduce systemic inflammation?
In the pilot experiments, we hypothesized that the stimulation of the cervical vagus nerve in ChAT-Cre x ChR2 mice with the Nerve Cuff 2.0 design would attenuate pro-inflammatory cytokines (IL-6, TNF-⍺, and IL-1β) and CRP. Previous literature has shown vagus nerve stimulation attenuates the production of these pro-inflammatory cytokines (IL-6, TNF-⍺, and IL-1β). CRP is commonly used as a clinical marker of inflammation with higher levels of the protein indicating higher severity of inflammation. The experiment was conducted as described in Figure 4.
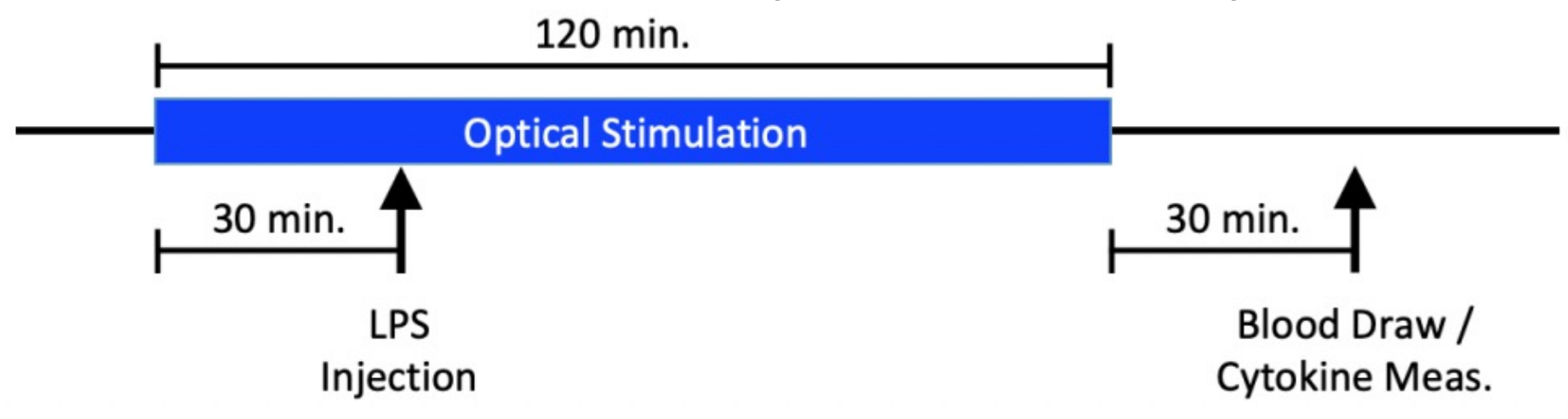
Figure 4: Pilot experiment structure. ChAT-Cre x ChR2 mice had Nerve Cuff 2.0 implanted around their cervical vagus nerve. Afterward they were optically stimulated using the optical fiber for 120min. At 30 minutes, the mice were injected with 2mg/kg of LPS. 30 minutes after the stimulation, blood was drawn from the mice and the cytokines levels were measured.
These pilot experiments were conducted on 4 mice, 2 controls and 2 experimental. With such a small sample size, the results are preliminary and incomplete. Our results, shown in Figure 5, indicate that TNF-⍺ and IL-1β attenuated with VNS. IL-6 levels were measured outside of the range of the assay, so the results of this marker are unclear. Finally, CRP resulted in increased levels with VNS, which contradicts the initial hypothesis.
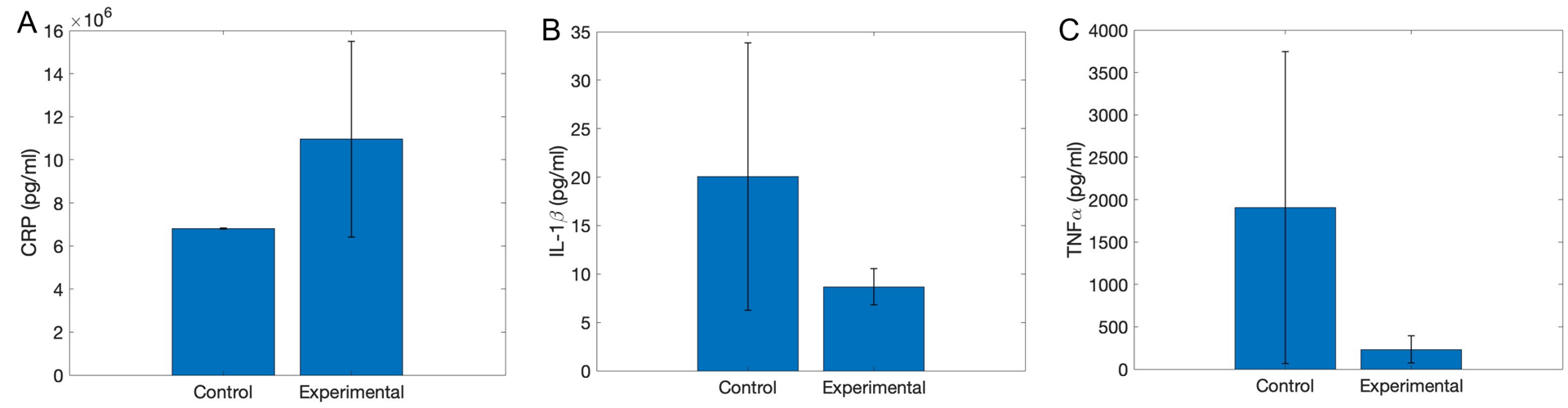
Figure 5: Serum concentrations of A: CRP, B: IL-1β, and C: TNF-⍺in control (sham surgery and cuff implant) and experimental (VNS) groups. Error bars depict standard error. N=2 per group.
What's next in our research?
- We will be continuing our experiments to determine the full effects of optical vagus nerve stimulation on inflammatory markers.
- We will be considering improvements that could be made to our experiment design:
- Optimizing the nerve cuff for easier implantation around the cervical vagus nerve
- Adjust the assay protocol to ensure all concentrations are within range of the assay
- Refinement of stimulation parameters
References
- Bonaz, B., Sinniger, V., Hoffmann, D., Clarençon, D., Mathieu, N., Dantzer, C., Vercueil, L., Picq, C., Trocmé, C., Faure, P., Cracowski, J. L., & Pellissier, S. (2016). Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neurogastroenterology and motility, 28(6), 948–953. https://doi.org/10.1111/nmo.12792.
- Borovikova, L. V., Ivanova, S., Zhang, M., Yang, H., Botchkina, G. I., Watkins, L. R., Wang, H., Abumrad, N., Eaton, J. W., & Tracey, K. J. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature, 405(6785), 458–462. https://doi.org/10.1038/35013070
- Caravaca, A. S., Gallina, A. L., Tarnawski, L., Tracey, K. J., Pavlov, V. A., Levine, Y. A., & Olofsson, P. S. (2019). An Effective Method for Acute Vagus Nerve Stimulation in Experimental Inflammation. Frontiers in neuroscience, 13, 877. https://doi.org/10.3389/fnins.2019.00877
