Vagnozzi Lab
Immuno-Fibrosis Signaling Mechanisms in Cardiac Pathophysiology
About the Vagnozzi Lab
Mission: Our lab studies cellular mechanisms of injury and stress responses in the heart. We are particularly interested in the innate immune system and how inflammation impacts wound healing, fibrosis, and tissue remodeling in beneficial versus adverse ways.
Approach: We use the mouse as our primary model, which allows us to interrogate gene functions and signaling pathways, perform genetic lineage tracing, and mimic complex cardiovascular pathology in a mammalian system. Our goal is to understand the regulatory mechanisms of cardiac wound healing and discover novel approaches to repair or even rejuvenate the damaged heart. Our approach is driven by a shared lab culture of teamwork, discovery, and high-quality science.
Ronald J. Vagnozzi, Ph.D.
Assistant Professor
Department of Medicine, Division of Cardiology
Gates Institute
Consortium for Fibrosis Research Translation (CFReT)
Functions of tissue macrophage subsets in cardiac health and disease
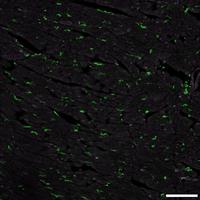
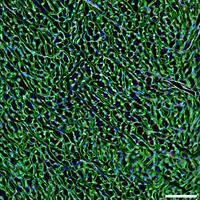
Signaling pathways regulating damage sensing and inflammation

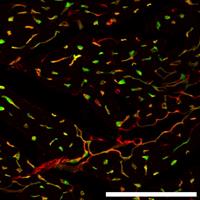
Crosstalk between immune cells, fibroblasts, and the extracellular matrix
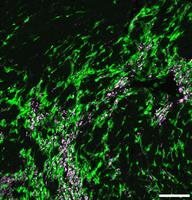
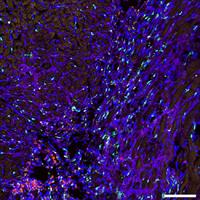
Genetic lineage tracing and genome-wide screening as discovery platforms
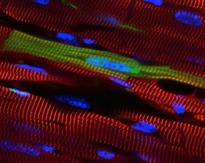
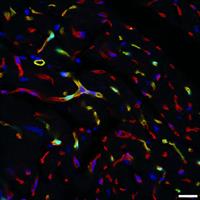

Current Lab Members:
Ronald J. Vagnozzi, Ph.D.

Assistant Professor
Department of Medicine, Division of Cardiology
Gates Institute
Consortium for Fibrosis Research Translation (CFReT)
Devon "Koya" Conradson, B.A.

Koya is currently a Ph.D. student in the Cell Biology, Stem Cells and Development (CSD) graduate program. He received a Bachelor's degree in Biochemistry and Molecular Biology from Lewis and Clark College. Prior to joining the lab, Koya worked as a summer intern at the SLAC National Accelerator Laboratory and as a Research Assistant in the Department of Neurosurgery at Stanford University.
Rachael Keeney, M.S.


Rachael is currently a Senior Professional Research Assistant in the UC Denver Pre-Clinical Cardiovascular Core. Rachael oversees the Vagnozzi lab mouse colony and provides support on animal husbandry, breeding and use of transgenic mice, and experimental design. Rachael received a Master's degree in Biology and Behavioral Ecology from Eastern New Mexico University.
Caroline Reges, M.S.

Caroline is currently a Professional Research Assistant and the lab manager for the Vagnozzi lab. Caroline received Bachelor's degrees in Integrative Animal Biology and World Languages and Cultures from the University of South Florida, and a Master's degree from Miami University in Biology - studying cold-induced cardiac remodeling across mammalian physiology.
Lab Alums:
Ilaria Ferrari, B.S.

Ilaria moved to Colorado in 2018 from New York City after receiving a B.S. in mechanical engineering from Columbia University. She conducted clinical research at Children's Hospital Colorado and then worked as a Professional Research Assistant in the Vagnozzi Lab until 2023. Ilaria is currently pursuing MD/PhD training at the University of Louisville.
Doron Regev, M.S. rLATG

Doron moved to Colorado from Boston, MA in June 2021. Doron received his B.S. in Biology from the University at Albany and a master's degree in Molecular Genetics and Microbiology from the University of Florida. Doron worked as a Professional Research Assistant and mouse colony manager for the Vagnozzi lab until 2022 before transitioning into the biotech industry.
Joseph Adjei

Joseph did a summer internship in 2021 through the Gates Center for Regenerative Medicine Summer Internship Program (GSIP). He is an international student studying Biomedical Science, Chemistry and Psychology at Rochester Institute of Technology, RIT.
Yifei Chen

Yifei did two summer internships in 2022 and 2023 through the Gates Center for Regenerative Medicine Summer Internship Program (GSIP). Yifei is currently a rising senior at Johns Hopkins University, majoring in Molecular Cellular Biology and Public Health Studies.
Shahad Al Wuhaili

Shahad did a summer internship in 2023 through the CUSP Summer Students Programs at the Webb-Waring Center. Shahad is currently a Sophomore in the class of 2026 at the University of Notre Dame, majoring in Chemical Engineering.
Mark Afanasyev

Mark did a dual-lab summer internship (Vagnozzi and McKinsey labs) in 2023 through the GEMS- Graduate Experiences for Multicultural Students program. Mark is currently a Sophomore in the Class of 2026 at Carnegie Mellon University, majoring in Chemistry.
Alenca Harrington, Ph.D.

Alenca conducted a fellowship in the Vagnozzi lab in 2023 after training in Medical Biochemistry, Genetics, and Epigenetics in Wales, UK and at the Sorbonne Université in Paris, France. She earned her PhD in 2023 from the University of Montpellier and INSERM, studying fibroblast activity in the context of heart failure and regeneration using zebrafish and iPS cells.
Lab Publications
2023:
Vagnozzi RJ, Robinson EL. Frataxin Deacetylation in Macrophages: Avoiding SIRTain Myocyte Death. Circ Res. 2023 Sep 15;133(7):648-650. doi: 10.1161/CIRCRESAHA.123.323503. Epub 2023 Sep 14. PMID: 37708247; PMCID: PMC10506396.
Emerging epigenetic therapies of cardiac fibrosis and remodelling in heart failure: from basic mechanisms to early clinical development. Cardiovasc Res. 2023;118(18):3482-3498. doi:10.1093/cvr/cvac142
Chromatin Remodeling Drives Immune-Fibroblast Crosstalk in Heart Failure Pathogenesis. Preprint. bioRxiv. 2023;2023.01.06.522937. Published 2023 Jan 7. doi:10.1101/2023.01.06.522937
2022:
T cell immunotherapy for cardiac fibrosis: mRNA starts the CAR. Cell Stem Cell. 2022;29(3):352-354. doi:10.1016/j.stem.2022.02.002
2021:
Cardiac Cell Therapy Fails to Rejuvenate the Chronically Scarred Rodent Heart. Circulation. 2021;144(4):328-331. doi:10.1161/CIRCULATIONAHA.120.053080
Mechanisms and strategies for a therapeutic cardiac immune response. J Mol Cell Cardiol. 2021 May 26:S0022-2828(21)00107-3. doi: 10.1016/j.yjmcc.2021.05.013. Epub ahead of print. PMID: 34051237.
2020:
SupErbB monocytes? Innate immune cells help the heart adapt. J Mol Cell Cardiol. 2020 Dec 11;152:92-94. doi: 10.1016/j.yjmcc.2020.12.003.
Resident macrophages keep mitochondria running in the heart. Cell Res. 2020 Dec;30(12):1057-1058. doi: 10.1038/s41422-020-00427-z.
Cardiac Cell Therapy Rejuvenates the Infarcted Rodent Heart via Direct Injection but Not by Vascular Infusion. Circulation. 2020 Mar 24;141(12):1037-1039. doi: 10.1161/CIRCULATIONAHA.119.044686.
An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. 2020 Jan;577(7790):405-409. doi: 10.1038/s41586-019-1802-2.
2019:
Basic Cardiovascular Sciences Scientific Sessions 2019: Integrative Approaches to Complex Cardiovascular Diseases. Circ Res. 2019 Oct 25;125(10):924-931. doi: 10.1161/CIRCRESAHA.119.315977.
CARdiac Immunotherapy: T Cells Engineered to Treat the Fibrotic Heart. Mol Ther. 2019 Nov 6;27(11):1869-1871. doi: 10.1016/j.ymthe.2019.09.021.
2018:
Genetic Lineage Tracing of Sca-1+ Cells Reveals Endothelial but Not Myogenic Contribution to the Murine Heart. Circulation. 2018 Dec 18;138(25):2931-2939. doi: 10.1161/CIRCULATIONAHA.118.035210.
New Myocyte Formation in the Adult Heart: Endogenous Sources and Therapeutic Implications. Circ. Res. 2018 Jul 6;123(2):159-176. doi: 10.1161/CIRCRESAHA.118.311208.
Lab Events!
Farewell party for Ilaria Ferrari

2023 Summer Intern Bowling Outing
