McKinsey Lab
Our lab is focused on understanding the signaling and gene regulatory mechanisms that control heart failure and associated disorders. We are particularly interested in the role of epigenetics in regulating the pathological cardiac hypertrophy and fibrosis
that is associated with heart failure. Nuclear DNA is wound around proteins called histones to form chromatin, and post-translational modification of histones represents one epigenetic mechanism for altering gene expression. Among the enzymes that
target histones are histone deacetylases (HDACs), histone acetyltransferases (HATs) and histone methyltransferases. We use molecular biology, biochemistry and pharmacology to address the roles of these and other epigenetic modifiers in the control
of gene expression in the heart, and extend our findings to surgical, transgenic and gene knockout models of heart failure. Our animal model studies involve echocardiographic and catheter-based measurements of heart function.
We are also interested in the mechanisms whereby signals derived from cell surface
receptors are conveyed to histone-modifying enzymes by proteins kinases and phosphatases. The long-term goal of our work is to translate basic discoveries to novel therapies for patients with heart failure, which afflicts millions of adults in the
U.S. and is associated with a 5-year mortality rate of nearly 50%. As such, our lab has established core expertise to enable in vitro, cellular and in vivo assessment of experimental small molecule compounds in support of early stage drug discovery.
Our lab emphasizes teamwork and camaraderie, thus creating an exciting environment for students and postdoctoral trainees.
I also co-direct the Consortium for Fibrosis Research & Translation (CFReT); CFReT.org.

Timothy A. McKinsey, Ph.D.
School of Medicine, Division of Cardiology
University of Colorado Denver
Anschutz Medical Campus
12700 E. 19th Ave
Aurora, CO 80045-0508
Tel: (303) 724-5476
[email protected]










Cardiac Physiology

Cardiac Fibrosis
Cardiac Hypertrophy
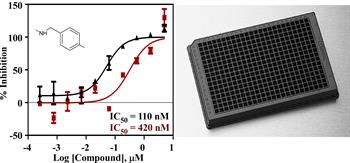
Pharmacology and High Throughput Chemical Biology
Signaling and Gene Regulation
McKinsey Lab Holiday Party 2021

2019 McKinsey Lab Holiday Lunch at Texas de Brazil

McKinsey Lab 2019

2019 Birthday Lunch

2018 Tim's 50th

2017 Hiking in Boulder

2017 FASEB Meeting in Montana

2017 Lab Outing: Rockies Baseball Game




2016 Holiday Christmas Dinner




2024
Gravi-D peptide disrupts HDAC11 association with an AKAP to stimulate adipocyte thermogenic signaling https://pubmed.ncbi.nlm.nih.gov/38690735/
2023
Inhibition of Eicosanoid Degradation Mitigates Fibrosis of the Heart https://pubmed.ncbi.nlm.nih.gov/36475698/
Reading a Good Transcript Soothes MYZAPed Heart https://pubmed.ncbi.nlm.nih.gov/37791296/
HDAC11 inhibition triggers bimodal thermogenic pathways to circumvent adipocyte catecholamine resistance https://pubmed.ncbi.nlm.nih.gov/37607030/
Substrate stiffness modulates cardiac fibroblast activation, senescence, and proinflammatory secretory phenotype https://pubmed.ncbi.nlm.nih.gov/37889253/
2022:
Therapeutic targets for cardiac fibrosis: from old school to next-gen https://pubmed.ncbi.nlm.nih.gov/35229727/
HDAC6 modulates myofibril stiffness and diastolic function of the heart https://pubmed.ncbi.nlm.nih.gov/35575093/
Arterial wall rejuvenation: the potential of targeting matrix metalloprotease 2 to treat vascular aging https://pubmed.ncbi.nlm.nih.gov/35512358/
Tissue is the issue: Endomyocardial biopsies to elucidate molecular mechanisms and tailor therapy for HFpEF https://pubmed.ncbi.nlm.nih.gov/35660295/
Targeting a transcriptional scleraxis to treat cardiac fibrosis https://pubmed.ncbi.nlm.nih.gov/36342270/
COVID-19 and BRD4: a stormy and cardiotoxic bromo-romance
Histone deacetylase 6 inhibition restores leptin sensitivity and reduces obesity
Reversible lysine fatty acylation of an anchoring protein mediates adipocyte adrenergic signaling
2021:
Cat-apulting Toward a Molecular Understanding of HFpEF
2020:
ERRing on the Side of a Mature Heart
The black sheep of class IIa: HDAC7 SIKens the heart
HDAC inhibition improves cardiopulmonary function in a feline model of diastolic dysfunction
2019:
Dynamic Chromatin Targeting of BRD4 Stimulates Cardiac Fibroblast Activation
Physiological Biomimetic Culture System for Pig and Human Heart Slices
Putting the Heat on Cardiac Fibrosis: Hsp20 Regulates Myocyte-To-Fibroblast Crosstalk
Gold Nanoparticle-Functionalized Reverse Thermal Gel for Tissue Engineering Applications
The Fibrosis Across Organs Symposium: A Roadmap for Future Research Priorities
Epigenetic therapies in heart failure
HDAC5 catalytic activity suppresses cardiomyocyte oxidative stress and NRF2 target gene expression
2018:
HDAC11 suppresses the thermogenic program of adipose tissue via BRD2
Epigenetics in Cardiac Fibrosis: Emphasis on Inflammation and Fibroblast Activation
Histone deacetylase governs diastolic dysfunction through a nongenomic mechanism.
2017:
Class I HDACs control a JIP1-dependent pathway for kinesin-microtubule binding in cardiomyocytes
p38α A Profibrotic Signaling Nexus
BRD4 inhibition for the treatment of pathological organ fibrosis.
2016:
The potential of targeting epigenetic regulators for the treatment of fibrotic cardiac diseases.
Epigenetic regulation of cardiac fibrosis.
2015:
Transgenic over-expression of YY1 induces pathologic cardiac hypertrophy in a sex-specific manner.
Non-sirtuin histone deacetylases in the control of cardiac aging.
AKT Network of Genes and Impaired Myocardial Contractility During Murine Acute Chagasic Myocarditis.
2014:
Tubulin hyperacetylation is adaptive in cardiac proteotoxicity by promoting autophagy.
BET-ting on chromatin-based therapeutics for heart failure.
Targeting cardiac fibroblasts to treat fibrosis of the heart: focus on HDACs.