Development of Novel Adeno-Associated Vectors (AAVs) for Targeting of Somatosensory Neural Subsets
Why is sensory feedback in prosthetic systems important?
Greater than 50% of upper-limb prosthetic users reject and abandon their prostheses, and one common factor is the lack of sensory feedback and the increased cognitive load associated1. When surveyed, over eighty percent of upper-limb prosthetic users detailed that grip force and feeling contact with objects was an extremely important feature desired in prostheses2, with similarly high results for movement and position feedback.
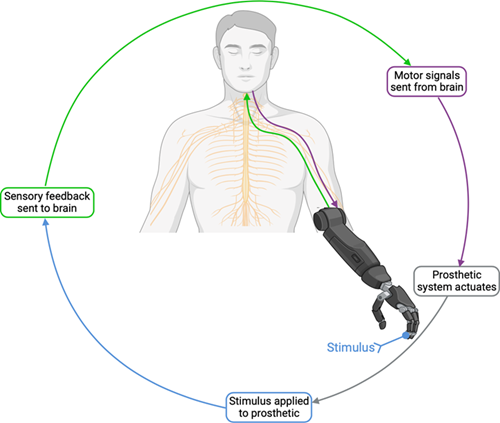
Figure 1: A simplified, ideal prosthesis control loop.
Why are current sensory feedback methods inefficient?
There are three primary categories of sensory feedback in the periphery; nociception, proprioception, and mechanoreception. Multiple methods to reintroduce sensory feedback have been explored, from auditory or vibratory feedback as an indicator of grip force, to electrical nerve stimulation. However, none of these methods accurately replicate a “natural” sense of feedback; mainly due to lack of specificity in stimulation. For example, electrical stimulation results in an “all-or-nothing” stimulation pattern to the nerves within the stimulation field. Given that peripheral nerves contain a combination of both motor and sensory neurons, with multiple subclasses of neurons within each, too much information is conveyed.
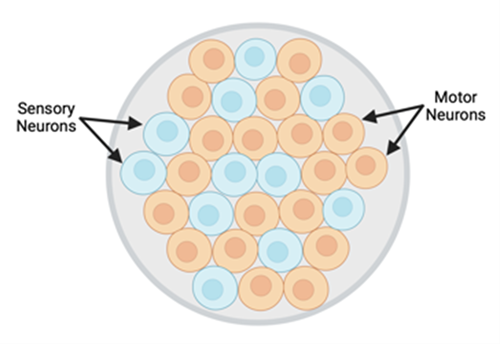
Figure 2: Transverse schematic of peripheral nerve; made up of both motor and sensory neurons.
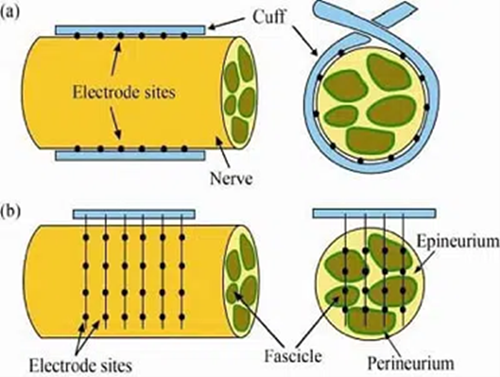
Figure 3: Schematic of electrical nerve cuffs (a) and electrical nerve arrays (b).
How optogenetics can address issues in current sensory feedback?
Optogenetics is the use of genetic editing to create light-responsive tissues through the introduction of optical proteins. Utilizing optogenetic techniques in neurons provides a method to monitor and control neural activity in a precise manner; both in ex-vivo environments and in freely moving animals3. Through the introduction of different optical proteins into neural systems, nerve activity can be stimulated, inhibited, or observed at a single axon level. Introducing these optical proteins into the peripheral nervous system will allow for a direct interface into motor control and the somatosensory system4, providing novel opportunities for advancements in prosthetic systems.
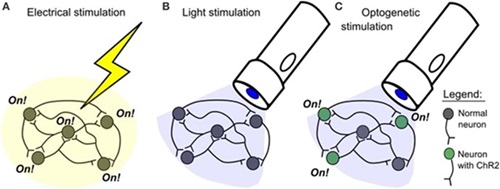
Figure 4: Comparison of electrical and optogenetic stimulation.
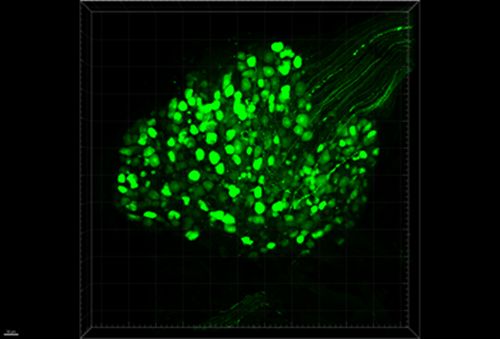
Figure 5: DRG cell bodies and axons expressing GFP.
How can we target the somatosensory system using optogenetics?
To introduce channelrhodopsins or GECIs into the peripheral nervous system (PNS), a method to genetically alter the neurons to express the optical proteins is necessary. Viral vectors can be customized based on virus, serotype, and promoter to achieve an extremely specific targeting construct. Adeno-associated vectors (AAVs) are one of the most used viral constructs in optogenetic experiments. With multiple serotypes, both natural and engineered5, providing individual tropisms for various cells and tissues6,and the further specificity that can be provided using cell-specific promoters, an enormous number of possible targeting constructs can be designed. All sensory neurons from the periphery are routed through the dorsal root ganglion (DRG), so finding the AAV serotype with a tropism for DRGs and inserting a cell-specific promoter will target the desired neurons7, specifically the somatosensory neurons in the periphery.
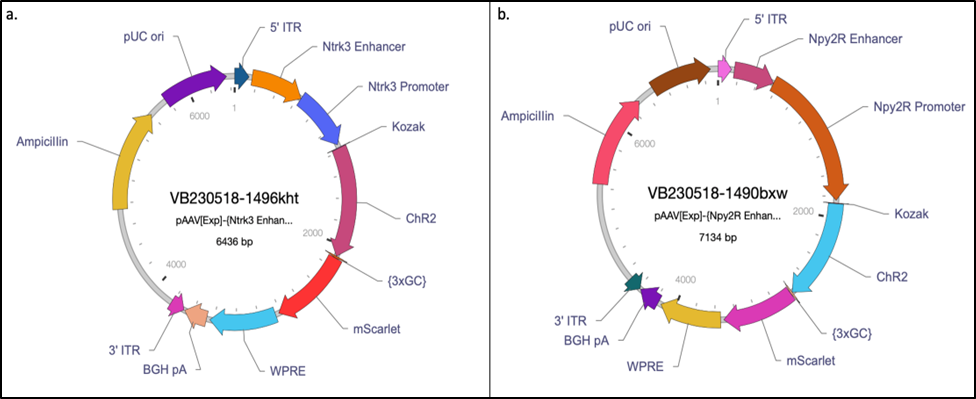
Figure 6: Final designs for a) Ntrk3 and b) Npy2R custom AAVs.
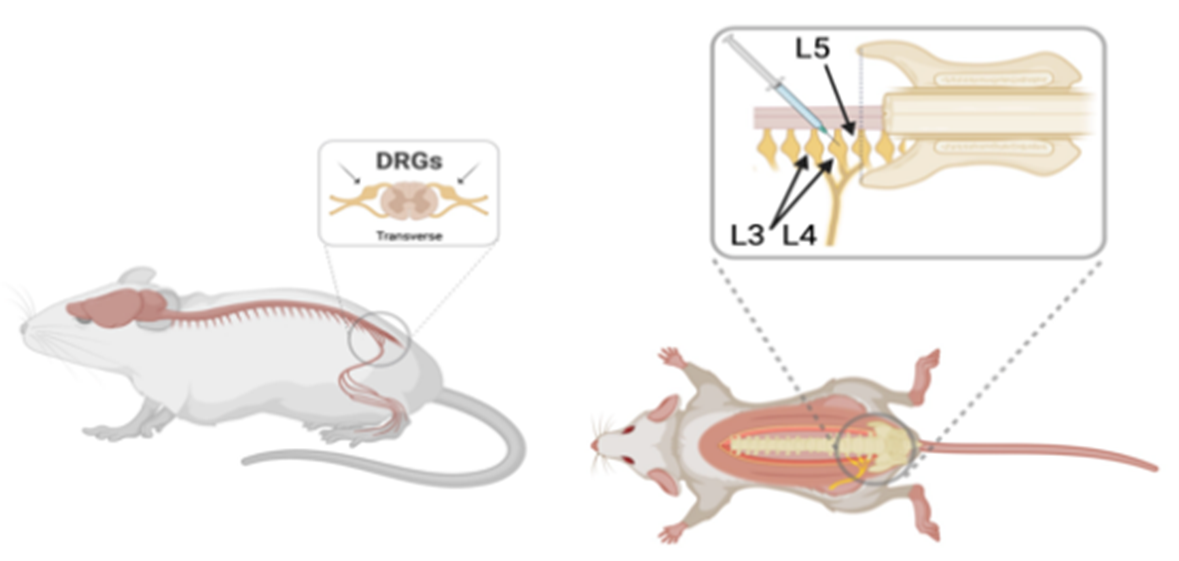
Figure 7: Lateral and dorsal view of where AAV is injected.
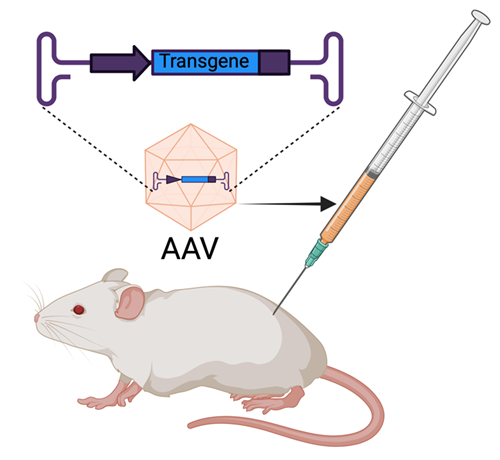
Figure 8: AAVs are delivered via injection.
References
1. Salminger, S., et al. (2022). "Current rates of prosthetic usage in upper-limb amputees - have innovations had an impact on device acceptance?" Disabil Rehabil 44(14): 3708-3713.
2. Lewis, S., et al. (2012). User demands for sensory feedback in upper extremity prostheses. 2012 IEEE International Symposium on Medical Measurements and Applications Proceedings: 1-4.
3. Yizhar, O., et al. (2011). "Optogenetics in neural systems." Neuron 71(1): 9-34.
4. Towne, C., et al. (2013). "Optogenetic control of targeted peripheral axons in freely moving animals." PLoS One 8(8): e72691.
5. Chan, K. Y., et al. (2017). "Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems." Nat Neurosci 20(8): 1172-1179.
6. Zincarelli, C., et al. (2008). "Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection." Mol Ther 16(6): 1073-1080.
7. Haenraets, K., et al. (2017). "Spinal nociceptive circuit analysis with recombinant adeno-associated viruses: the impact of serotypes and promoters." J Neurochem 142(5): 721-733.
