Instrumentation and Services
Analytical ultracentrifugation relies on the simple premise that biomolecules in solution will sediment in the presence of the gravitational force applied by the centrifuge. The rate at which the particle sediments will be affected by its molecular weight, size and shape allowing one to determine these parameters experimentally. There are two basic types of experiments performed with the analytical ultracentrifuge, sedimentation velocity and sedimentation equilibrium.
Sedimentation velocity experiments rely on a high angular velocity which causes the solute to sediment rapidly leading to a depletion of solute near the meniscus. A boundary forms between the depleted and uniform concentration areas of the solute which can be monitored to determine the sedimentation coefficient which is a measure of the effective size of the solute.
Sedimentation equilibrium experiments employ a smaller angular velocity than sedimentation velocity which causes the solute to sediment at a much slower rate. As sedimentation occurs, diffusion opposes the gradual concentration increase in the bottom of the cell. After the two opposing forces reach equilibrium, the diffusional flow exactly balances the sedimentation flow leading to a concentration profile that is constant over time from which the molecular weight of the solute can be determined.
Applications
- Examination of Sample Purity
- Molecular Weight Determination
- Analysis of Associating Systems
- Detection of Conformational Changes
- Ligand Binding
- References (click at left for links)
Instrument Info
- XL-A (Beckman Coulter, Installed 1999)
- Rotor: An-60 Ti - 4-place titanium rotor rated for 60,000 rpm
- An-Ti 50 – 8-place titanium rotor rated for 50,500 rpm
- XL-I (Beckman Coulter, Installed 2005)
Sample Requirements
Sedimentation Velocity: At least 500uL of sample with an OD of 0.7-0.8 at the scanning wavelength. Bring at least 5 mL of buffer as well.
Sedimentation Equilibrium: At least 500uL of sample with an OD of 0.2-0.3 at the scanning wavelength. Bring at least 10mL of buffer as well.
Training
Training is administered by the staff of the biophysics core and must be completed in advance of any experiments.
References
Web sites of interest to Optima(tm) XL-A/XL-I Analytical Ultracentrifuge users.
- Reversible Associations in Structural and Molecular Biology (RASMB). The website at the Boston Biomedical Research Institute offers archived software, tutorials for time derivative and Nonlin software, bibliographies, links, and FAQs.
- Center for Analytical Ultracentrifugation of Macromolecular Assemblies (CAUMA) at the University of Texas Health Sciences Center, San Antonio. Publications by the faculty, tutorials for Van Holde-Weischet software, and educational and diagnostic information.
- Protein Interactions Core, University of Utah. Provides information about analytical ultracentrifugation, titration calorimetry and optical biosensor technologies, which are used to define the assembly state, thermodynamics and kinetics of a reaction. The facility also operates on a fee-for-service basis.
- Association of Biomolecular Research Facilities (ABRF). News, electronic posters, discussion groups concerning major technologies.
- National Analytical Ultracentrifugation Facility of the University of Connecticut Biotechnology Center. Offers software, collaborative studies, modified instrumentation and workshops.
- UK National Centre for Macromolecular Hydrodynamics (NCMH). Consultation and collaborative work using analytical ultracentrifugation and other techniques.
- Iowa State University Protein Facility
Plane polarized light is made up of a left-handed (L) and a right-handed component (R) which are equal in magnitude. Circular Dichroism (CD) refers to the difference in absorption of these two components by a sample. Only chiral chromophores will produce a CD signal which can occur for the following reasons:
- An intrinsically chiral chromophore such as a carbon atom bonded to four different ligands or a disulphide bond with chirality because of dihedral angles in its C-S-S-C chain of atoms.
- Chromophores covalently bonded to a chiral center.
- Chromophores placed in an asymmetric environment due to the 3D structure of the molecule
If the sample does not absorb or equally absorbs both the L and R components, then the resulting radiation should be polarized in the original plane. However, if circular dichroism occurs, than the resulting radiation will posses an elliptical polarization.
CD instruments generally measure this elliptical polarization (ellipticity) in terms of degrees (θ):
θ = tan-1 (b/a)
b = minor axis of the resultant ellipse
a = major axis of the resultant ellipse
Optical rotatory dispersion (ORD) is a technique used to study the chirality of a biomolecule and consists of passing a beam of linearly polarized light through the sample. If there is any degree of chirality in the sample, then the light will be rotated as a function of wavelength and from this rotation, one can determine the chirality (left- or right-handed) of the molecule.
Applications
- Protein/Peptide conformational studies
- Thermal and chemical denaturation studies
- Structure determination
Instrument Info
J815 (Jasco, Installed Feb. 2010). The instrument includes a Peltier temperature control device for thermal denaturations. Optical rotatory dispersion (ORD) capability.
Sample Requirements
- 0.1cm sample cell: 200uL of sample
- 0.2cm sample cell: 300uL of sample
- Larger cells are available on request and you are welcome to bring your own quartz cells
- For Far UV Measurements (190nm-250nm):
- Peptides: 0.1mg/mL (Typical)
- Nucleic Acids: 0.5 OD (Typical)
- For Near UV (250nm-350nm)
- Peptides: 1mg/mL
Training
Training consists of a brief walkthrough of the instrument and its operating procedures with core facility personnel. This is required before any experiments can be performed.
To get a basic feel for the procedure of the experiment, it is recommended that you review this tutorial before coming in to use the instrument.
- Circular Dichroism Procedure
- Temperature Melt Procedure
- CD Fluorescence Procedure
- Optical Rotary Dispersion Procedure
Conversions & Prediction Programs
- See the Biophysics Core Handbook for conversions and other handy information used in CD studies.
- K2D M.A. Andrade, P. Chacón, J.J. Merelo, and F. Morán, European Molecular Biology Laboratory, Heidelberg, Germany.
The K2D method uses a self-organising neural network to extract the secondary structure features present in the data from a set of circular dichroism spectra ranging from 200 nm to 241 nm.
The network is trained using a number of reference spectra and the information is stored in a matrix for recall. k2d then uses this pre-calculated data to predict the secondary structures present based on your CD data. The run does not take more than a few seconds and provides an estimation of the percentages of helix, sheet and random structure of your protein. k2d also gives the probable error in the estimation, based on its training procedure.
- ANTHEPROT (ANalyse THE PROTeins) is the result of about 10 years of biocomputing activity of a group of the Institute of Biology and Chemistry of Proteins. The main idea was to integrate into a single package most of the methods designed for protein sequence analysis (1,2,3,4). This package contains a number of analysis routines that predict secondary structure based on CD data.
- DICROPROT (DICHROism of PROTeins) is from the same group as ANTHEPROT and integrates most of the methods designed for the estimation of protein sequence secondary structure derivation from circular dichroism experiments into a single package. The major goal in making DICROPROT is to help the user to manage the spectra acquired with Jobin-Yvon apparatus.
- CD 222:208 ratio 1.10 => Coiled coil
CD 222:208 ratio 0.90 = > Helix
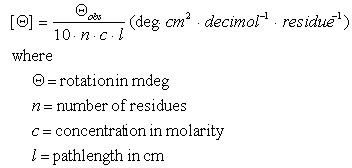
Molar Ellipticity Online Conversion
Surface Plasmon Resonance is a phenomenon generated by the incidence of polarized light onto an electrically conductive surface at the interface between it and a solution with different bulk refractive indices. This will only occur if the incident light contacts the surface at such an angle as to cause total internal reflection. Part of the light will be reflected while another part of the light will be absorbed by the electrons on the surface and propagate as an electric field across the surface. This electric field is known as the evanescent wave. The angle of the light such that a minimum amount of reflected light is observed is known as the resonance angle and is directly proportional to refractive index of the solution adjacent to the surface. Biacore instruments measures the resonance angle and the variations in this angle caused by changes in refractive index of the solution. As mass binds to the surface, it modifies the bulk refractive index of the solution causing the angle of the reflected light minima to change leading to a measurable signal. The amount by which the resonance angle changes is directly proportional to the mass bound. Molecular interactions are thus studied by immobilizing one binding partner onto the gold sensor surface (known as the ligand) and using these surfaces to capture the other binding partner from solution (known as the analyte).
Applications
There are a wide variety of experiments possible with the Biacore, some of which include:
- Kinetics Analysis of Molecular Interactions
- Concentration Measurement
- Activity Assays
- Binding Site Analysis
- Screening of Interaction Partners
Instrument Info
Biacore3000 (Biacore, Installed Sep. 2000)
Temperature Range: 20-30°C (Instrument is generally run at 25°C)
Sample Requirements
Each user is required to use their own Sensor Chip for their experiments and these may either be purchased from Biacore directly or from the facility. These sensor chips come with a variety of surface chemistries suitable for specific applications and allow for the immobilization of ligand through a wide variety of coupling chemistries. A careful literature search is generally the best method to determine what concentration of sample will be required for both the ligand and the analyte as this will vary greatly from experiment to experiment. Generally concentrations in the 0.1-1mg/mL are needed for the ligand and uM or nM concentrations for the analyte depending on the strength of the interaction.
Training
Training involves the completion of one experiment under the supervision of Core Facility personnel. The experiment can be one of the trainee’s choosing.To get a basic feel for the procedure of the experiment, it is recommended that you review the tutorial before coming in to use the instrument.
pdf
Biacore 3000 Tutorial
(pdf)
261 KB
Differential Scanning Calorimetry (DSC) is a biophysics technique that can be used to determine enthalpies associated with various temperature induced transitions. A DSC instrument basically measures the amount of heat required to raise the temperature of the sample (i.e. the heat capacity) over a temperature range. If the sample undergoes a phase transition, the heat capacity of the sample will fluctuate in relation to a reference cell producing a peak in the data. The area of this peak then corresponds to the enthalpy of that transition (ΔH).
Information Available
Enthalpy values (ΔH) for transitions and binding interactions, Gibb’s Free Energy Values (ΔG) and Heat Capacity Values (C).
Applications
- Comparison of Protein Stabilities
- Detection of Weak/High Affinity Binding to Proteins
- Determination of Protein Oligomerization
- Thermodynamic Structural Information
Instrument Info
VP-DSC (MicroCal, Installed Jan. 2001)
Short Term Noise (RMS average from 5 °C to 110 °C): 1 µcal/min (0.07 uwatts)
Baseline Repeatability (successive scans from 5 °C to 110 °C):
2.5 µcal/min (0.2 uwatts)
Minimum Response Time: 5 seconds
Operating Temperature Range: -10 to 130 °C
Maximum Scan Rate – Heating: 90 °C/hour
Maximum Scan Rage – Cooling:
60 °C/hour
Scanning Rate: Heating: 0 - 90 °C/hour
Cooling: 0 - 60 °C/hour
Effective Volume:
0.5 ml in a tantalum alloy cell. Stem volume is about 0.085 ml.
Pressurized Chamber: 0 - 30 psi. Can reach to 80 psi with accessory pressure cap.
Signal: Exothermic events will cause
the DP signal to deflect in the negative direction. Vice versa for endothermic reactions.
Data Acquisition: 16 bit A/D
Sample Requirements
Approximately 0.5mL is the total volume of the cell but ~1mL is needed for loading the cell. Additionally, bring 5-10mL of buffer to run blanks and fill the reference cell.
Training
Training involves the completion of one experiment under the supervision of Core Facility personnel. The experiment can be one of the trainee’s choosing.
To get a basic feel for the procedure of the experiment, it is recommended that you review the tutorial before coming in to use the instrument.
Isothermal titration Calorimetry (ITC) is a powerful tool used to determine the thermodynamics of a particular reaction between two molecules. ITC experiments are performed at constant temperature by titrating one binding partner (the titrant) into a solution containing the other partner (the titrand). After each aliquot of titrant is added, the heat absorbed or released by its reaction with the titrand is measured with respect to a reference cell containing buffer. The heat change is measured in electrical power (J/s) as it is the difference in power needed to maintain the sample and reference cells at two similar temperatures.
Information Available from ITC
Binding Constant (Kb), Reaction Stoichiometry (n), Heat of Reaction (ΔH) and Entropy of Reaction (ΔS).
Applications
- Small Molecule-Protein Interaction Thermodynamics
Instrument Info
ITC200 (microCal, Installed 2013), VP-ITC (Microcal, Installed Jan. 2001)
Sample Requirements
ITC200: Cell Volume: 300uL; Syringe Volume - 80uL
VP-ITC: Cell Volume: 2mL; Syring Volume - 300uL
Concentration Requirements: Generally, the minimum cell concentration is 10uM and the syringe concentration should be 10-20x that of the cell concetration. Keep in mind every experiment is unique and if you have any questions, please consult with the facility manager before beginning an experiment.
Training
Training involves the completion of one experiment under the supervision of Core Facility personnel. The experiment can be one of the trainee’s choosing.
Fluorescence is a luminescence caused by the emission of a photon upon the relaxation of an electron in an excited state to its ground state. Fluorescence spectroscopy, the detection of this phenomenon, is a powerful technique for biological applications as it can be used quantitatively and is a more sensitive technique than absorbance. The wide variety of fluorescent molecules available commercially makes this technique widely applicable to many systems.
Applications
- Quantitative detection of biomolecules
- Studies of biomolecular structure
Instrument Info
Fluoromax-3 (Horiba Jobin Yvon, Installed May 2005)
Both the Polarizer and Plate Reader Accessories are installed on this instrument.
Sample Requirements
The standard rectangular cell takes approximately 1mL to fill.
The 96-well plates for the reader are available for purchase from the core and each well has a volume of ~200uL
Users are welcome to bring and use their own cuvettes.
Training
Training consists of a basic walkthrough of the instrument conducted by core facility personnel.
Microscale Thermophoresis (MST) is a technique that is used to quantitatively measure binding using changes in thermophoretic mobility as an indicator. The instrument will heat one spot in the cell causing molecules to diffuse across the temperature gradient (thermophoresis) and will measure the distribution of molecules using fluorescence. If two molecules in solution interact with one another, they will move differently in a temperature gradient and this difference is proportional to the amount bound. The advantage of this instrument over other methods of studying binding, such as surface plasmon resonance or isothermal titration calorimetry, is that the sample requirements are up to 100-fold less, can be used with a variety of buffer systems and relies on fluorescence, which is easily incorporated into most systems.
Instrumentation Available
Nanotemper Monolith NT.115 Pico (Red/Blue)
Pico RED Detector
- Minimum Fluorophore Concentration: > 50pM
- Range of KD Measured: pM- mM
- Excitation Wavelengths: 600-645nm
- Example Fluorophores: Cy5, NT647, Alexa647
Nano BLUE Detector
- Minimum Fluorophore Concentration: > 5nM
- Range of KD Measured: nM- mM
- Excitation Wavelengths: 460-490nm
- Example Fluorophores: Alexa488, GFP, Fluorescein, NT495
Example References
The Biophysics Core is equipped with an Agilent 1260 Infinity.
Key features of Agilent 1260 Infinity:
- UV/Vis with optional Fluorescence detector
- Auto-sampler with space for 91 samples (up to 200 uL)
- 4 pumps for isocratic and gradient elution
- Column temperature control
- Sample injection volume from 1 – 200 uL
- Compatible with most HPLC columns
We also have a variety of columns to perform various separations including reverse phase, normal phase, and analytical size exclusion columns. Users are also able to provide/purchase their own columns for use as well. The core is well equipped for the analysis of everything from small molecules to proteins.
We can also help design and adapt an HPLC method for separations on our system. Please contact the Biophysics Core for setting up a method and sample runs.
Dynamic Light Scattering (DLS) is a technique that can be used to measure the size distribution of small particles in solution. Practical applications of this technique include determining protein homogeneity of a solution, assessing aggregation or oligomerization, determining protein stability, optimizing proteins for crystallization, and screening high-concentration protein solutions for reversible association and viscosity. The core is currently equipped with a Wyatt DynaPro Plate Reader III
Key features of DynaPro Plate Reader III:
- Measure hydrodynamic radius from 0.5 nm to 1000 nm
- Sensitivity for size down to 0.125 mg/mL lysozyme
- Measure weight-average molar mass from 1 to 1000 kDa
- Utilizes microwell plates of 96, 384 or 1536 wells
- Sample volume from 4 μL to 150 μL
- All measurements made in situ in the wells
- Temperature ramps from 4°C to 85°C for melting curve analysis
References
Structural Biology and Biophysics Core Facilities
School of Medicine
General
CMS Login