Robert Murphy, PhD
University Distinguished Professor Emeritus

Contact Information:
University of Colorado Denver
Department of Pharmacology
Mail Stop 8303, RC1-South
12801 East 17th Ave
Aurora CO 80045
Phone: (303) 724-3352
Fax: (303) 724-3657
E-mail: [email protected]
Office: RC1-Sorth, L18-6120

More information on Dr. Murphy's book, here
Past Research
Overview
While I have now retired from active, laboratory research I can summarize the research carried out for the past 45+ years in the 4 areas below. In general they focused on the basic biochemistry and pharmacological control of lipid mediators derived from both enzymatic and nonenzymatic pathways largely employing techniques of sophisticated mass spectrometry to address critical issues. The term lipid mediators is used here in the context that many of the compounds under investigation have potent and diverse biological activities that permit cells to intercommunicate with each other. For example, following exposure to reactive oxygen species new lipid molecules are formed from endogenous lipids by covalent alteration of their chemical structure and these new molecules cause cells in a tissue to respond to the oxidant stress. A major emphasis was to involve the use of mass spectrometry for both qualitative and quantitative investigations in this area of lipid biochemistry. Five specific areas the general focus of activities in this laboratory and they include:
- Leukotriene biosynthesis
- Transcellular biosynthesis of le ukotrienes
- Studies of eicosanoid metabolism and studies of non-enzymatic lipid oxidation
- Phospholipid remodeling and eicosanoid biosynthesis
- Mass spectrometric studies of lipids, lipidomics, and imaging mass spectrometry
1. My early scientific investigations centered largely on the structural characterization of a bioactive principle, then termed slow reacting substance of anaphylaxis. These structural studies culminated in 1979 with the characterization of this activity as a cysteinyl leukotriene while on sabbatical in the laboratory of Professor Bengt Samuelsson (Karolinska Institutet). This work expended our appreciation of the complexity of arachidonate metabolism and the description of the 5-lipoxygenase pathway of arachidonic acid that leads to a completely novel set of biologically active eicosanoids. These discoveries launched studies worldwide in this pathway, including the development of pharmacological agents to disrupt formation and action of these molecules. Also, in these early years an understanding of the biosynthesis of leukotrienes in pulmonary tissue was examined. In addition, I participated in the structural characterization of platelet activating factor as a unique ether phospholipid. Recently the seminar paper published in 1979 was reprinted as a “Pilar of immunology”.
- Orange RP, Murphy RC, Karnovsky ML, Austen KF. The physiochemical characteristics and purification of slow reacting substance of anaphylaxis. J Immunol 110:760-770, (1973).
- Murphy RC, Hammarstrom S, Samuelsson B. Leukotriene C: A slow reacting substance (SRS) from murine mastocytoma cells. Proc Natl Acad Sci USA 76:4275-4279 (1979). PMCID411556
- Stenmark KR, James SL, Voelkel NF, Toews WH, Reeves JT, Murphy RC. Leukotriene C 4 and D4 in neonates with hypoxemia and pulmonary hypertension. N Engl J Med 309:77-80 (1983).
- Clay KL, Murphy RC, Andres JL, Lynch J, Henson PM. Structure elucidation of platelet activating factor derived from human neutrophils. Biochem Biophys Res Commun 121:815-825 (1984).
- Murphy RC, Hammarström S, Samuelsson B. Pillars Article: Leukotriene C: A Slow-Reacting Substance from Murine Mastocytoma Cells. Proc. Natl. Acad. Sci. USA. 1979. 76: 4275-4279. J Immunol. 200:1538-1542 (2018).
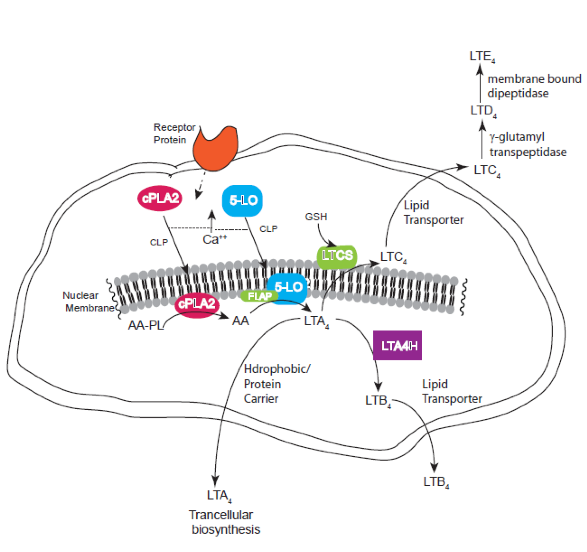
Figure 1. Biosynthesis of leukotrienes within stimulated cells. Increase in intracellular Ca++ leads to translocation of cytosolic phospholipase A2 (cPLA2a ) and 5-lipoxygenase (5-LO) to the nuclear membrane where esterified arachidonate (AA-PL) is transformed into free arachidonic acid (AA). This is the substrate for the FLAP/5-LO complex to convert AA to LTA4. Cells that express LTC4 synthase (LTCS) or leukotriene A4 hydrolase (LTAH) convert this reactive intermediate LTA4 into LTC4 or LTB4 respectively. These are then transported out of the cell. Extracellular metabolism leads to the production of LTD4 and LTE4.
2. The cooperation of cells in the biosynthesis of leukotrienes led to the discovery of a process termed transcellular biosynthesis where two or more cells cooperate to complete the entire biosynthetic cascade leading to the bioactive leukotrienes, leukotriene C4 and leukotriene B4. Neutrophils, when activated produce leukotriene B 4, however, when neutrophils are incubated in the presence of platelets and then activated, a major product is leukotriene C4. Platelets were found not to have 5-lipoxygenase, but contain leukotriene C4 synthase. These transcellular biosynthetic events were found to be surprising efficient in vivo using chimeric mice. The existence of this complexity of biosynthesis has permitted an understanding of the potential role of leukotrienes in pathology such as traumatic brain injury and retinopathy. Where neuroma cells which do not express 5-lipoxygenase can participate in the formation of leukotrienes.
- Maclouf J, Murphy RC. Transcellular metabolism of neutrophil-derived leukotriene A4 by human platelets. J Biol Chem 263:174-181 (1988).
- Maclouf J, Fitzpatrick FA, Murphy RC. Transcellular biosynthesis of eicosanoids. Pharmacol Res 21:1-7 (1989).
- Farias S, Frey LC, Murphy RC, Heidenreich KA. Injury-related production of cysteinyl-leukotrienes contributes to brain damage following experimental traumatic brain injury. J Neurotrauma 26:1977-1986 (2009). PMCID2822801
- Zarini S, Gijón MA, Ransome AE, Murphy RC, Sala A. Transcellular biosynthesis of leukotrienes in vivo during mouse peritoneal inflammation. Proc Natl Acad Sci USA 106:8296-8301 (2009). PMCID2688893
- Capra V, Rovati GE, Mangano P, Buccellati C, Murphy RC, Sala A. Transcellular biosynthesis of eicosanoid lipid mediators. Biochim Biophys Acta 1851:377-382 (2015).
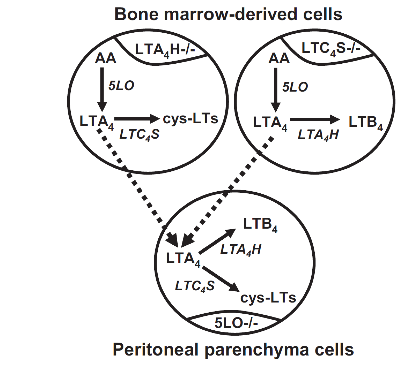
Figure 2: Experimental model of transcellular leukotriene biosynthesis. 5LO-/- mice were lethally irradiated, then rescued with bone marrow cells from LTA4H-/- or LTC4S-/- mice. Upon zymosan stimulation, cells of a bone marrow origin could synthesize either cysLTs (if donors were LTA4H-/-) or LTB4 (if donors were LTC4S-/-), but no single cell could synthesize both. Parenchymal cells could not synthesize either, since they did not express 5LO. To produce both types of LTs, LTA4 needed to be exported from bone marrow-derived cells and imported into parenchymal cells, then converted to LTB4 and cysLTs. LTA4H and LTC4S were both expressed in the parenchymal cell population, although not necessarily within the same cell.
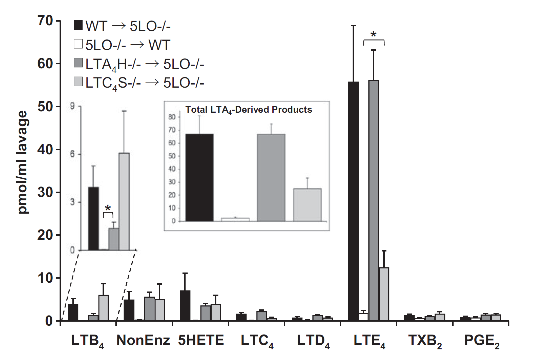
Figure 3. Production of eicosanoids in peritoneal lavage of chimeric mice. Chimeric mice (bone marrow donor → irradiated acceptor) were treated with 1 mg zymosan for 2 h, and the indicated eicosanoids were analyzed by LC/MS/MS and quantitated by isotopic dilution of the corresponding internal standards. The LTB4 section is expanded for clarity. The inset shows the sum of all LTA4-derived metabolites (Δ6-trans-LTB4 isomers and 5,6-diHETE isomers, LTB4 and cysLTs). Results are average ± SEM of at least three.
3. The structural characterization of metabolites of leukotrienes as well as prostaglandins had been investigated in cells, tissues, and in the intact animal which has led to a description of metabolites that can be used to assess production of leukotrienes and prostaglandins in vivo. Furthermore, these studies have revealed the important role of metabolism to terminate biological activity.
- Stene DO, Murphy RC. Metabolism of leukotriene E4 in isolated rat hepatocytes: Identification of β-oxidation products of sulfidopeptide leukotrienes. J Biol Chem 263:2773-2778 (1988).
- Shirley MA, Murphy RC. Metabolism of leukotriene B4 in isolated rat hepatocytes: Involvement of 2,4-dienoyl CoA reductase in leukotriene B4 metabolism. J Biol Chem 265:16288-16295 (1990).
- Maclouf J, Antoine C, De Caterina R, Sicari R, Murphy RC, Patrignani P, Loizzo S, Patrono C. Entry rate of leukotriene C4 into the vascular compartment and subsequent metabolism in healthy subjects. Am J Physiol 263:H244-H249 (1992).
- Zemski-Berry KA, Borgeat P, Gosselin J, Flamand L, Murphy RC. Urinary metabolites of leukotriene B4 in the human subject. J Biol Chem 278:24449-24460 (2003).
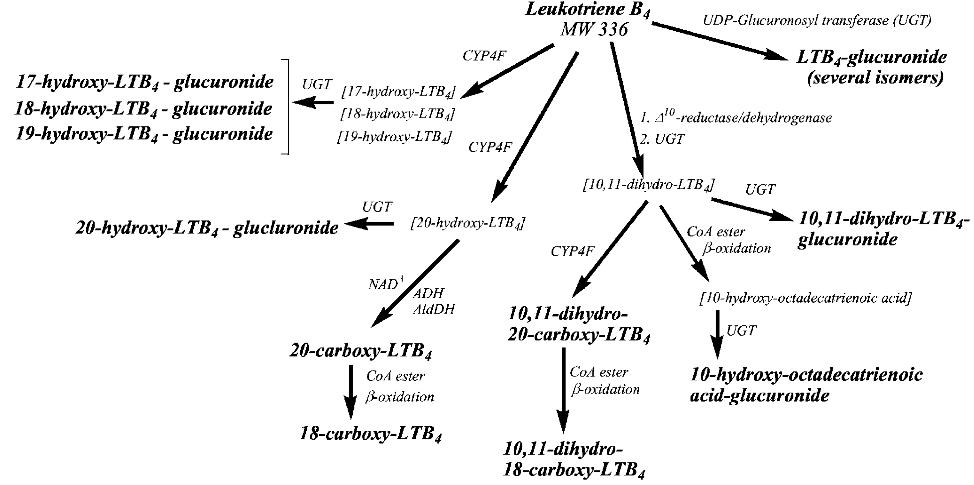
Figure 4. Proposed pathway for metabolism of LTB4 in the human subject with urinary metabolites identified in a human subject treated with exogenous LTB4, indicated. Required intermediate metabolites not observed are indicated in brackets. UDP-glucuronosyl transferase(UGT)-dependent pathways resulting in glucuronide metabolites and cytochromeP-450(CYP4F) pathways are indicated.
4. Fundamental studies of phospholipid biochemistry have also been a major focus of this laboratory. Initial studies led to a description of the rapid remodeling of arachidonic acid within the human neutrophil. Employing for the first time mass spectrometry to study molecular species of phospholipids. More recently, this interest in phospholipid biochemistry has led to the description of the lysophospholipid acyltransferases present in the human neutrophil and the use of a novel substrate choice assay based on mass spectrometry to study the four major lysophospholipid acyltransferases. In addition to this, many methods were developed based on electrospray tandem mass spectrometry to study molecular species of phospholipids which naturally led to the involvement of this laboratory with the Lipid MAPS consortium and a major initiative into the building of infrastructure for lipid biochemistry based on mass spectrometry.
- Chilton FH, Murphy RC. Remodeling of arachidonate-containing phosphoglycerides within the human neutrophil. J Biol Chem 261:7771-7777 (1986).
- Gijón MA, Riekhof WR, Zarini S, Murphy RC, Voelker DR. Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils. J Biol Chem 283:30235-30245 (2008). PMCID2573059
- Hankin JA, Barkley RM, Zemski Berry K, Deng Y, Murphy RC. Mass spectrometric collisional activation and product ion mobility of human serum neutral lipid extracts. Anal Chem 88:6274-6282 (2016). PMCID5007945
- Martin SA, Gijon MA, Voelker DR, Murphy RC. Measurement of lysophospholipid acyltransferase activities using substrate competition. J Lipid Res 55:782-791 (2014).
- O'Donnell VB, Murphy RC. Directing eicosanoid esterification into phospholipids. J Lipid Res. 58:837-839 (2017).
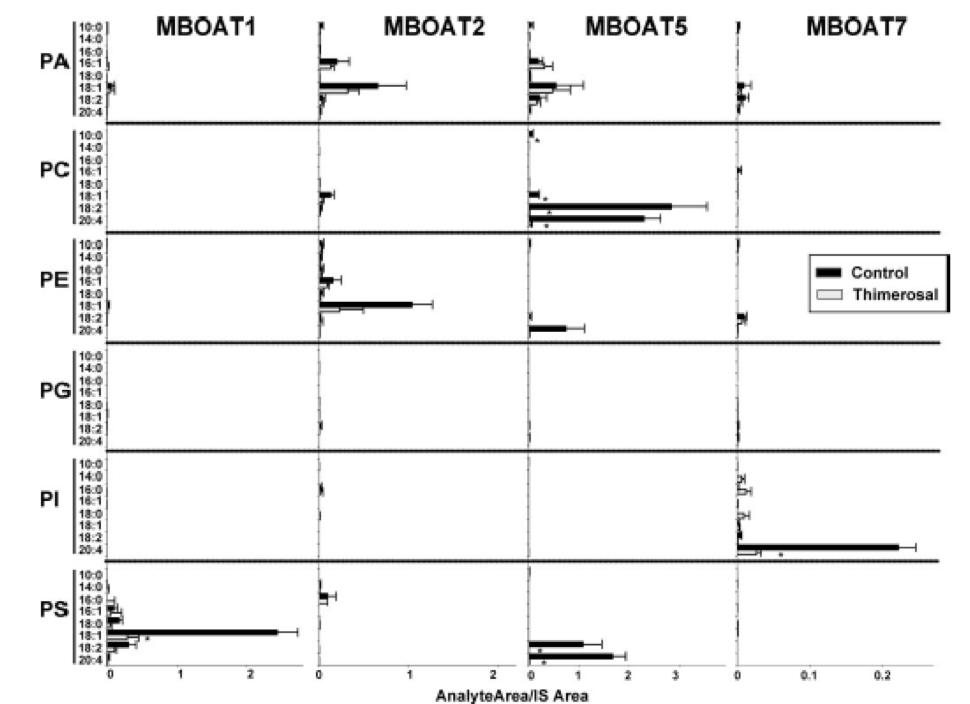
Figure 5. Dual substrate choice acyltransferase assay of microsomes from yeast expressing human MBOAT proteins. Acyltransferase was assayed as described above, with microsomes from yeast expressing the indicated human proteins, in the absence or presence of 50 µM thimerosal. The amount of each individual phospholipid species is expressed as the percentage of its integrated LC/MS/MS signal to the corresponding phospholipid internal standard added at the end of the incubation. Data are normalized for protein expression, and background activity present in yeast transformed with empty vector is subtracted. Data shown are average ± SEM of at least two individual experiments performed in triplicate.
5. A general interest in the tandem mass spectrometry of lipids has emerged over many years and many classes of lipids have been studied in terms of their gas phase ion chemistry. These include phospholipids, eicosanoids, neutral lipids, and lipid oxidation products. This includes a description of the complexity of oxidized cholesterol esters present in human atherosclerotic plaques. A major advance has been the use of imaging mass spectrometry based on MALDI ionization in a quadrupole time-of-flight mass spectrometer to identify and measure distribution of complex lipids in tissue slices such as distribution of phospholipids in brain regions.
- Okuno T, Gijon MA, Zarini S, Martin SA, Barkley RM, Johnson CA, Ohba M, Yokomizo T, Murphy RC. Altered eicosanoid production and phospholipid remodeling during cell culture. J Lipid Res. 2018 Jan 20. pii: jlr.M083030. doi: 10.1194/jlr.M083030. [Epub ahead of print]
- Zemski Berry KA, Barkley RM, Berry JJ, Hankin JA, Hoyes E, Brown JM, Murphy RC. Tandem mass spectrometry in combination with product ion mobility for the identification of phospholipids. Anal Chem 89:916-921 (2017). PMCID5250582
- Zemski Berry KA, Murphy RC. Phospholipid ozonation products activate the 5-lipoxygenase pathway in macrophages. Chem Res Toxicol 29:1355-1364 (2016). PMCID5029860
- Murphy RC. Tandem Mass Spectrometry of Lipids: Molecular Analysis of Complex Lipids. New Developments in Mass Spectrometry Series (S. Gaskell, Editor) Royal Society of Chemistry, London, UK (2015).
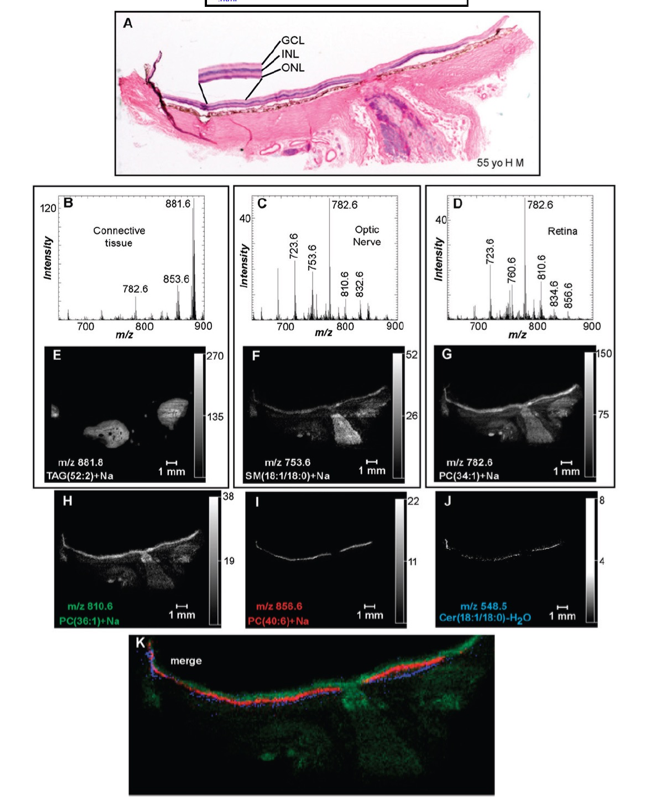
Figure 6. Positive ion MALDI IMS of PC and SM lipids in human ocular tissue. A: H&E stain of ocular tissue section immediately adjacent to the section used for MALDI imaging. Total positive ion MALDI mass spectra of the accessory tissue (B), optic nerve (C), and retina (D) were obtained directly from the ocular section. Extracted positive ion MALDI images of the [M+Na]+ of TAG(52:2) (m/z 881.8) (E), SM(d18:1/18:0) (m/z 753.6) (F), PC(34:1) (m/z 782.6) (G), PC(36:1) (m/z 810.6) (H), PC(40:6) (m/z 856.6) (I), and the [M+H-H2O]+ of Cer(d18:1/18:0) (m/z 548.5) (J). K: Merged positive ion MALDI image of Cer(d18:1/18:0) (blue), PC(40:6) (red), and PC(36:1) (green). GCL, ganglion cell layer.