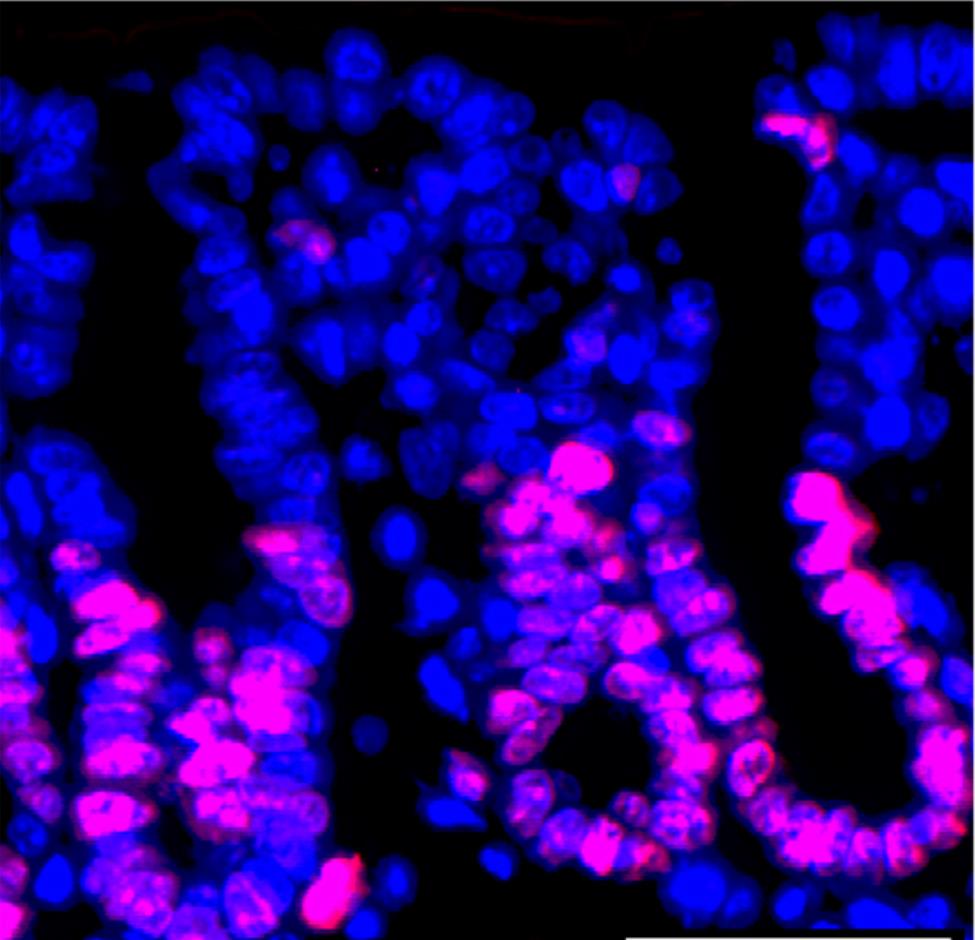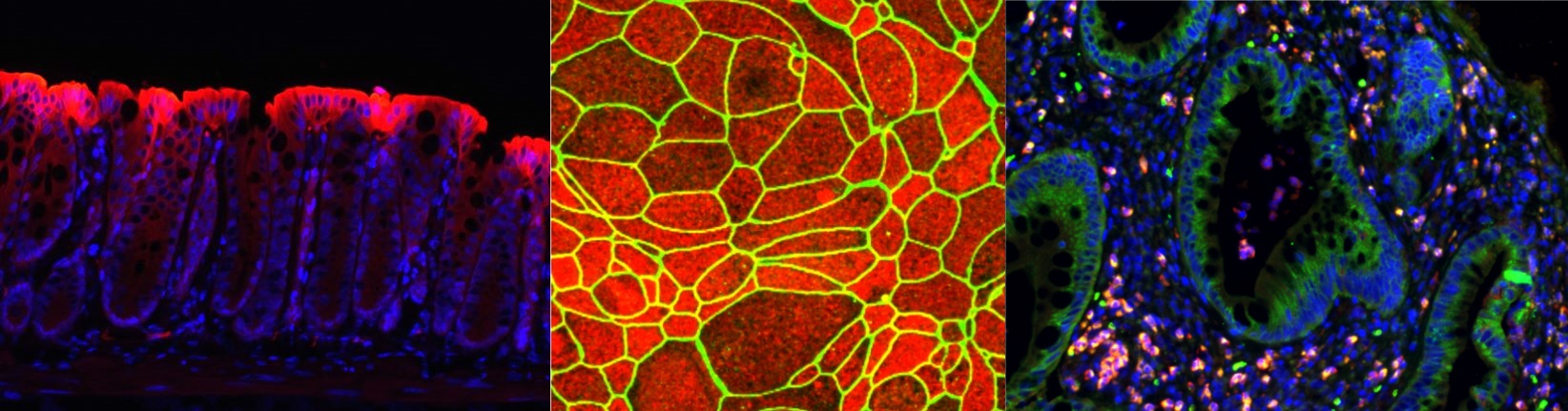
Sean Colgan Lab
 Overview
Overview
The Colgan Lab studies mucosal inflammation with focus on intestinal inflammation in the context of inflammatory bowel disease and other GI diseases. Studies are aimed at understanding how epithelial and endothelial cells coordinate barrier function and inflammatory responses at mucosal surfaces. Our lab takes a multifaceted approach by investigating the relationships between gut microbiota, host immune system, genetic background, and environmental influences as it pertains to mucosal health and disease, with research emphasis on energy metabolism, host-microbe interactions, hypoxia-inducible factor, and innate immunity.
Click here for a complete list of published work in Dr. Colgan's Bibliography.
Sean Colgan, Ph.D
Levine-Kern Professor of Medicine and Immunology
Sean Colgan Lab Staff
Ji Yeon Kim, Ph.D
| Inflammatory bowel disease (IBD) including Crohn’s disease and ulcerative colitis is a gastrointestinal disease characterized with a chronic and recurring inflammation. While the precise pathogenesis of IBD remains elusive, dysbiosis-an imbalance of gut microbiota-emerges as a common feature of IBD, accompanied by decreased levels of microbiota-derived metabolites. These metabolites have been implicated in the regulation of the immune response and the maintenance of gut homeostasis. In the Colgan lab, my research focuses on identifying potential microbial-derived metabolites that contribute to maintain intestinal homeostasis during inflammation and elucidating their underlying mechanisms. |
Rachel Cohen
| The intestinal epithelium exists in a state of "physiological hypoxia" or low oxygen under homeostatic conditions. My graduate work focuses on uncovering how hypoxia and the transcription factor hypoxia inducible factor (HIF) stabilization during hypoxia influence mucosal immunity. Specifically, I examine the role of hypoxia and HIF in the modulation of intestinal epithelial responses to IFNg and the role of HIF in T cell responses to intestinal inflammation. |
Jake Countess |
Noah Thompson | 
|

Barb Fox |
Alison Hinjosa |
Colgan Lab Contact:
12700 E.19th Ave. B146
P15-10450C
Research Complex 2
Aurora, CO 80045
Lab: (720) 724-7249
Colgan Lab Alumni
1994-1996 Gregor B. Zund, MD
1994-1996 Shoichi Uezono, MD
1994-1997 Andrea Dzus, BS
1996-1997 Gary B. Freidman, MD
1996-1998 Elizabeth D. Blume, MD
1995-1998 Steven J. Lisco, MD
1995-1999 Paul F. Lennon, MD
1996-2001 Cormac T. Taylor, PhD
1997-2000 Kristin Synnestvedt, BS
1997-2003 Glenn T. Furuta, MD
1999-2000 Kellie Park, MD, PhD
1999-2001 Katrina Comerford, PhD
1999-2002 Sailaja Narravula, MD
1999-2002 Donald W. Lawrence, PhD
2001-2003 Holger K. Eltzschig, MD
2000-2005 Jorn Karhausen, MD
2002-2004 Jonathon Phillips, PhD
2003-2004 Julio Morote-Garcia, Phd
2000-2006 Nancy Louis, MD
2002-2006 Tianqing Kong, MD
2001-2006 Geraldine Canny, PhD
2002-2006 Juan Ibla, MD
2005-2006 Andreas Robinson, PhD
2005-2006 Joseph Khoury, PhD
2005-2006 Peter Rosenberger, MD
2005-2009 Melanie Scully, PhD
2006-2008 Thomas Weissmueller, MD
2006-2011 Chris MacManus, PhD
2006-2011 Simon Keely, PhD
2006-2008 Karina Irizarry, MD
2008-2010 Jonathan Goldstein, MD
2010-2014 Caleb Kelly, MD, PhD
2011-2013 Stefan Ehrentraut, MD
2005-2010 Eric Campbell, PhD
2007-2012 Louis Glover, PhD
2007-2009 Mark Gerich, MD
2009-2013 Doug Kominsky, PhD
2009-2012 Brittelle Bowers, PhD
2010-2013 Joanne Masterson, PhD
2010-2013 Blair Fennimore, MD
2011-2014 Colm Collins, PhD
2012-2015 Dan Kao, MD, PhD
2013-2014 Kristi Kuhn, MD, PhD
2014-2016 Robert Isfort, MD
2012-2018 Valerie Curtis, PhD
2014-2018 Leon Zheng, MD, PhD
2015-2019 Erica Alexeev, PhD
2016-2018 Carlene Chun, MD
2016-2019 Carrie Hall, MD, PhD
2013-2021 Jordi Lanis, PhD
2016-2021 Ruth Wang, PhD
2017-2021 Rachel Gao, PhD
2018-2023 David Kitzenberg, MD, PhD
2006-2008 Karina Irizarry, MD
2008-2010 Jonathan Goldstein, MD
2010-2014 Caleb Kelly, MD, PhD
2011-2013 Stefan Ehrentraut, MD
2005-2010 Eric Campbell, PhD
2007-2012 Louis Glover, PhD
2007-2009 Mark Gerich, MD
2009-2013 Doug Kominsky, PhD
2009-2012 Brittelle Bowers, PhD
2010-2013 Joanne Masterson, PhD
2010-2013 Blair Fennimore, MD
2011-2014 Colm Collins, PhD
2012-2015 Dan Kao, MD, PhD
2013-2014 Kristi Kuhn, MD, PhD
2014-2016 Robert Isfort, MD
2012-2018 Valerie Curtis, PhD
2014-2018 Leon Zheng, MD, PhD
2015-2019 Erica Alexeev, PhD
2016-2018 Carlene Chun, MD
2016-2019 Carrie Hall, MD, PhD
2013-2021 Jordi Lanis, PhD
2016-2021 Ruth Wang, PhD
2017-2021 Rachel Gao, PhD
2018-2023 David Kitzenberg, MD, PhD