William J. Betz, PhD
Professor Emeritus
/william-betz----100-x-130.jpg?sfvrsn=65062b9_4)
Department of Physiology and Biophysics
University of Colorado School of Medicine
RC1 North Tower, P18-7113
Mail Stop 8307
Aurora, CO 80045
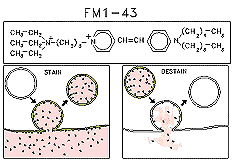 In the early 1990’s, we developed and characterized fluorescent dyes (called “FM dyes”) that have proven useful for selective staining of recycling endosomes,
secretory granules, and especially synaptic vesicles in a variety of cell types. When an endosome forms in the presence of the dye, its internal leaflet contains dye molecules, which are trapped inside. If the extracellular dye is washed away, labeled
endosomes remain as the only florescence signal. Finally, if the endosome (say, a recycling synaptic vesicle) undergoes exocytosis, its dye us spilled and the fluorescence signal disappears.
In the early 1990’s, we developed and characterized fluorescent dyes (called “FM dyes”) that have proven useful for selective staining of recycling endosomes,
secretory granules, and especially synaptic vesicles in a variety of cell types. When an endosome forms in the presence of the dye, its internal leaflet contains dye molecules, which are trapped inside. If the extracellular dye is washed away, labeled
endosomes remain as the only florescence signal. Finally, if the endosome (say, a recycling synaptic vesicle) undergoes exocytosis, its dye us spilled and the fluorescence signal disappears.
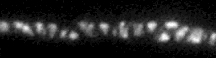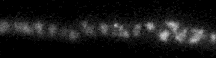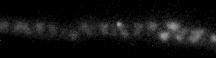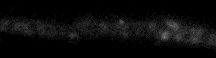 Frog motor nerve terminals (2-3 um in diameter) stain in a punctate fashion. Each spot is a cluster of several hundred stained synaptic vesicles. During repetitive
stimulation, they destain in a few minutes. The experiment illustrated in this time-lapse movie lasted 3.5 minutes; the nerve was stimulated repetitively at 30 Hz. (If the movie stops, press 'Reload'.)
Frog motor nerve terminals (2-3 um in diameter) stain in a punctate fashion. Each spot is a cluster of several hundred stained synaptic vesicles. During repetitive
stimulation, they destain in a few minutes. The experiment illustrated in this time-lapse movie lasted 3.5 minutes; the nerve was stimulated repetitively at 30 Hz. (If the movie stops, press 'Reload'.)
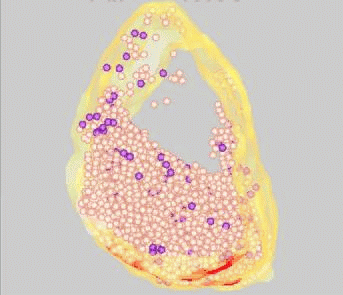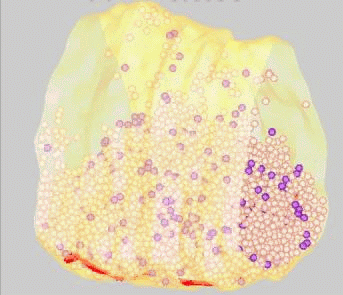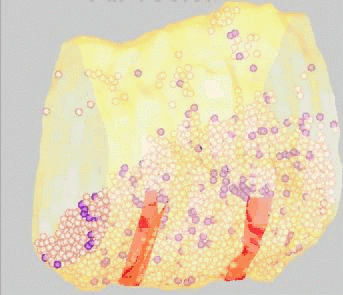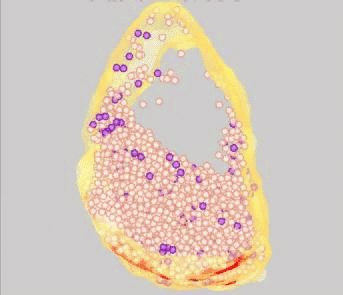 FM dyes can be photo-converted, that is, rendered visible in the electron microscope. We used this technique to map positions of synaptic vesicles belonging to the Readily-Releasable
Pool of vesicles. These are the first to undergo exocytosis during repetitive nerve stimulation. Surprisingly, these vesicles (purple) are not clustered near release sites (red), but rather are scattered among the population of vesicles that belong
to the Reserve Pool (white).
FM dyes can be photo-converted, that is, rendered visible in the electron microscope. We used this technique to map positions of synaptic vesicles belonging to the Readily-Releasable
Pool of vesicles. These are the first to undergo exocytosis during repetitive nerve stimulation. Surprisingly, these vesicles (purple) are not clustered near release sites (red), but rather are scattered among the population of vesicles that belong
to the Reserve Pool (white).
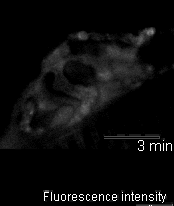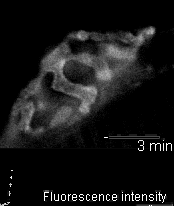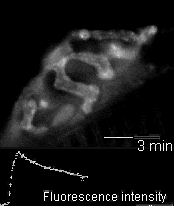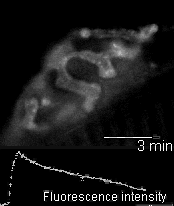 In 2005 we were invited to collaborate with Lucia Tabares and colleagues in Seville, Spain, in characterizing a transgenic mouse that they made, which expresses synaptopHluorin (spH - click here for details). Its motor nerve terminals fluoresce
when stimulated. The movie shows a single terminal. It was stimulated at 100 Hz for 10 seconds, causing it to grow brighter. It dimmed again when the stimulation ended as the spH was requenched. This preparation provides many new opportunities to
investigate presynaptic function and synaptic vesicle recycling.
In 2005 we were invited to collaborate with Lucia Tabares and colleagues in Seville, Spain, in characterizing a transgenic mouse that they made, which expresses synaptopHluorin (spH - click here for details). Its motor nerve terminals fluoresce
when stimulated. The movie shows a single terminal. It was stimulated at 100 Hz for 10 seconds, causing it to grow brighter. It dimmed again when the stimulation ended as the spH was requenched. This preparation provides many new opportunities to
investigate presynaptic function and synaptic vesicle recycling.
Cano, R., Torres-Benito, L., Tejero, R., Biea, A.I., Ruiz, R., Betz, W.J., Tabares, L (2012). Structural and Functional Maturation of Active Zones in Large Synapses. Molecular Neurobiology, In Press.
Cano, R., Ruiz, R., Shen, C., Tabares, L., and Betz, W.J. (2012) The functional landscape of a presynaptic terminal. Cell Calcium, In Press.
Hoopmann, P., Rizzoli, S.O., and Betz, W.J. (2012) FM dye photoconversion for visualizing synaptic vesicles by electron microscopy. Cold Spring Harb Protoc. 1, 84-86. PMID 22194271.
Hoopmann, P., Rizzoli, S.O., and Betz, W.J. (2012) Imaging synaptic vesicle recycling by staining and destaining vesicles with FM dyes. Cold Spring Harb Protoc.1, 77-83. PMID 22194270.
Gaffield, M.A., Romberg, C.F., and Betz, W.J. (2011) Live imaging reveals stochastic growth of bulk endocytic structures in frog motor nerve terminals. J. Neurophysiol., 106, 599-607. (cover illustration)
Ruiz, R., Cano, R., Casañas, J.J., Gaffield, M.A., Betz, W.J., and Tabares, L. (2011). Active zones and the readily releasable pool of synaptic vesicles at the neuromuscular junction of the mouse. J. Neurosci., 31, 2000 - 2008.
Michael A. Gaffield, Lucia Tabares and W. J. Betz (2009). The spatial pattern of exocytosis and post-exocytic mobility of synaptopHluorin in mouse motor nerve terminals. J. Physiol. 587, 1187-2000 (cover illustration).
Johnson, J.M., and Betz, W.J. (2008). The color lactotroph secretory granules stained with FM1-43 depends upon dye concentration. Biophys. J., 94, 3167-3177.
Gaffield, M.A. and Betz, W.J. (2007). Synaptic vesicle mobility in mouse motor nerve terminals with and without synapsin. J. Neurosci., 27, 13691-13700.
Tabares, L., Ruiz, R., Linares-Clemente, P., Gaffield, M.A., Alvarez de Toledo, G., Fernandez-Chacon, R., Betz, W.J. (2007). Monitoring synaptic function at the neuromuscular junction of a mouse expressing synaptopHluorin. J.Neurosci., 27, 5422-5430.
Gaffield, M.A., and Betz, W.J. (2007). Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nature Protocols, 1, 2916-2921.
Rizzoli, S.O. and Betz, W.J. (2005) Synaptic Vesicle Pools. Nature Rev. Neurosci 6, 57-70 (cover illustration).
Rizzoli, S.O. and Betz, W.J. (2004) The structural organization of the readily releaseable pool of synaptic vesicles. Science. 203, 2037-2039.
Richards, D.A., Guatimosim, C., Rizzoli, S.O., and Betz, W.J. (2003). Synaptic vesicle pools at the frog neuromuscular junction. Neuron 39, 529-541.
Cochilla, A.J., Angleson, J.K., and Betz, W.J. (2000) Differential regulation of granuel-to-granule and granule-to-plasma membrane fusion during secretion from rat pituitary lactotrophs. J. Cell Biol., 150, 829-848.
Betz, W.J. & G.S. Bewick. (1992). Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science 255: 200-203. (cover illustration)