Alon Poleg-Polsky, MD, PhD
Assistant Professor
/alon-poleg-posky----200-x-200.jpg?sfvrsn=b96411b9_4)
Department of Physiology and Biophysics
University of Colorado Anschutz
RC1 North Tower, P18-7101
Mail Stop 8307
Aurora, CO 80045
Tel (303) 724-3882
E-mail: [email protected]
Shared Content Block:
Physiology & Biophysics Styles -- smart float
Our brains process a wide variety of sensory and internally-generated signals with marvelous spatial and energetic efficiency. In the lab, we seek to describe the mechanisms that enable neural circuits to efficiently detect, amplify and transmit relevant information under diverse physiological conditions. This knowledge is required to understand how the healthy brain works and to develop strategies to treat abnormal neurological conditions. To achieve this goal, we combine advanced experimental techniques, computer modeling, and statistical analyses to study signal processing in single cells and neural networks in the retina, where neuronal and network dynamics can be studied in the well-defined context of visual processing.
Ongoing Projects
1. Changes in retinal function in disease
We examine how traumatic brain injury (TBI) and glaucoma models (optic nerve crush, ONC and microbead injection) affect visual processing. Our goal is to find predictors of resilience to damage and develop treatments to preserve retinal ganglion cell (RGC) function.
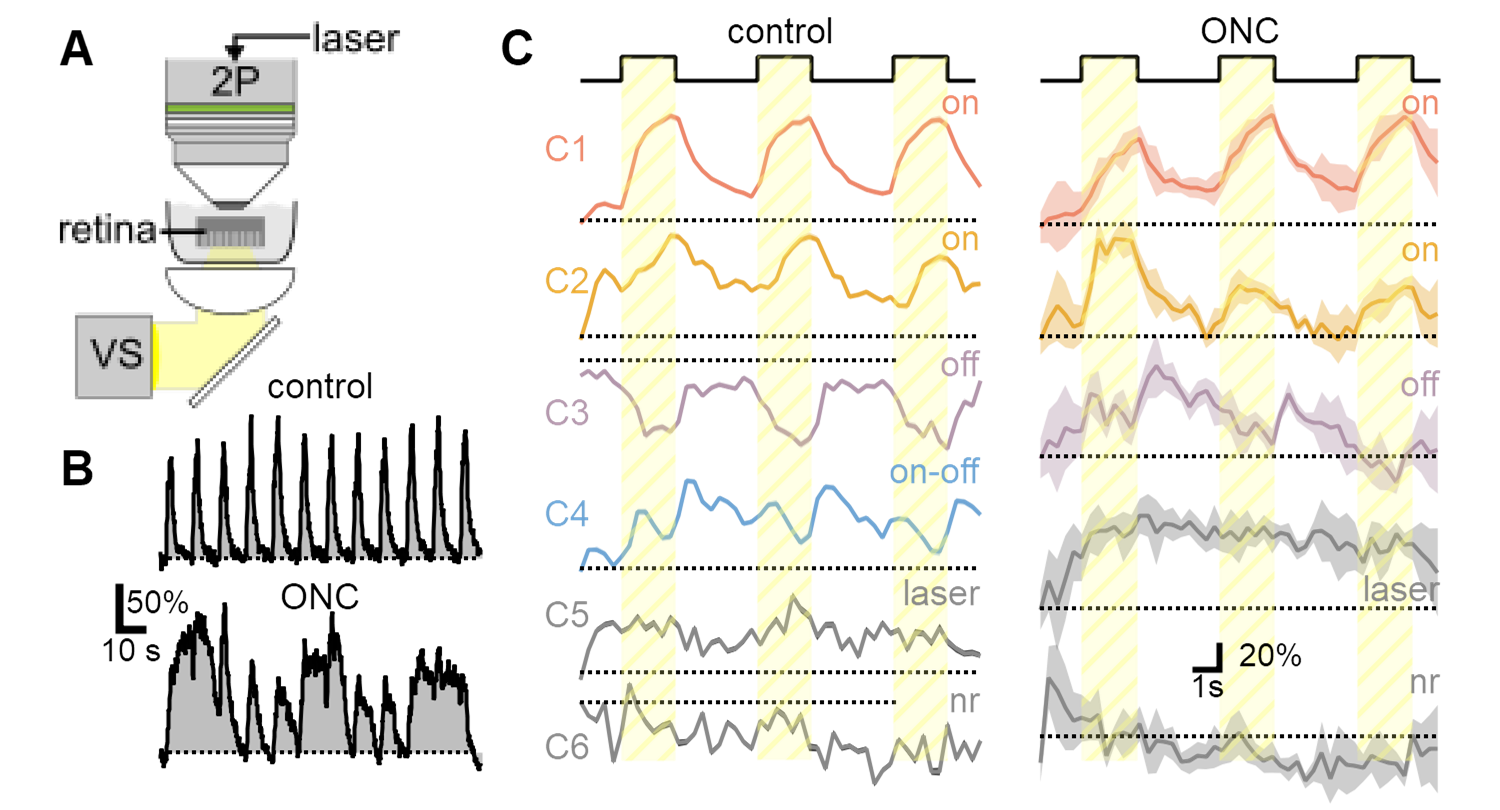
Abnormal RGC function in ONC glaucoma model. A) Simultaneous visual stimulation and two-photon microscopy in ex-vivo retina. B) Representative response dynamics from a single RGC to a repetitive stimulation show evidence of hyperexcitability after model induction. C) Diversity of visual responses observed in OPN4-Cre/GCaMP6 line is diminished after nerve crush, corresponding to the loss of susceptible RGC populations.
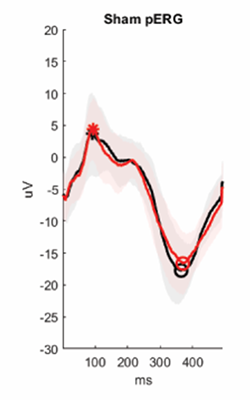
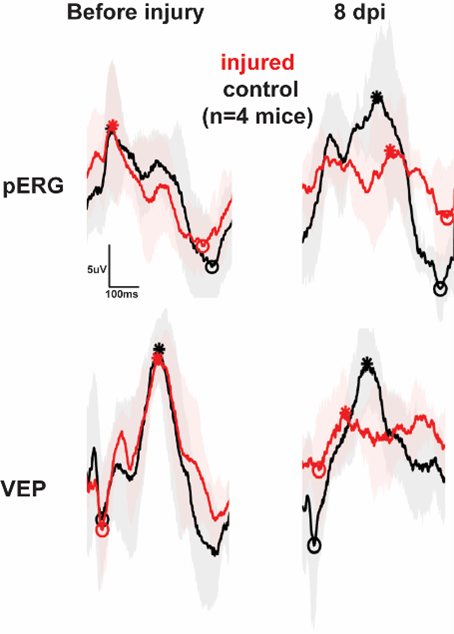
Pattern electroretinogram (PERG) performed before (black) and after (red) TBI or sham manipulation. Stars and circles indicate the peak and the trough of the responses. The figure illustrates the detrimental impact of brain injury on visual function.
2. Machine-learning-assisted receptive field mapping
REVEAL (Receptive field EValuation using Evolutionary ALgorithm) uses a genetic algorithm-based approach to reconstruct the receptive field of visual neurons. REVEAL is composed of a population of candidate solutions with center (excitatory) and surround (inhibitory) spatial maps, which are integrated into the full receptive field.
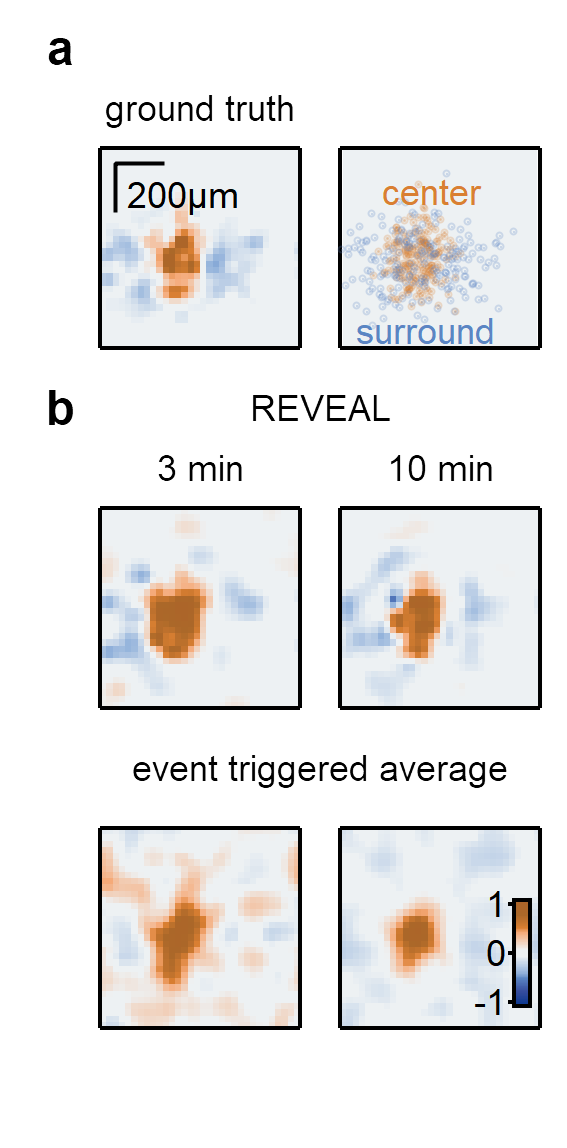
a) Example synthetic RF (left) and the distribution of the synaptic inputs (right). b) Top, RF maps produced by REVEAL based on responses to 3- and 10-minute-long super-resolution binary noise stimuli. Bottom, maps calculated with an event-triggered average approach from the same dataset.
3. Spatial distribution of ganglion cells in the mouse retina
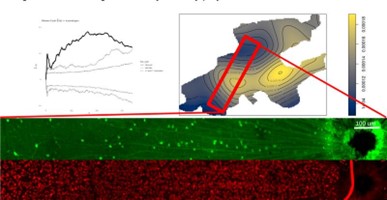
Spatial transcriptomics techniques are used in the lab to explore the organization of retinal neurons in normal conditions as well as changes that occur after an insult (traumatic brain damage and glaucoma). The figure shows a Monte Carlo simulation of VGlut3-GCaMP cells (GFP stain in green) demonstrating statistically significant spatial clustering relative to all ganglion cells (RBPMS stain from the same region in red).
4. Mechanisms of Direction Selectivity in the retina
The first step of direction selectivity (DS) is computed in the dendrites of starburst amacrine cells (SACs). A long-standing question in the field is how SACs detect and transmit directional information. Using detailed models and recordings from SAC dendrites, we expand and refine the range and properties of possible mechanisms by which this computation takes place. In several projects, we investigate the contribution of presynaptic inputs in generating DS. Based on detailed numerical simulations of synaptic integration in SACs dendrites we hypothesize that distinct classes of voltage-gated channels, known to be present in SACs, can form dendritic micro-domains with different rules for synaptic release. We aim to show that these dendritic channels can enhance the computation of DS, and even generate DS signals de-novo.
We develop biologically-inspired computer models of the DS circuit. Our goal is to perform a Systematic exploration of novel circuit mechanisms that can compute direction selectivity.
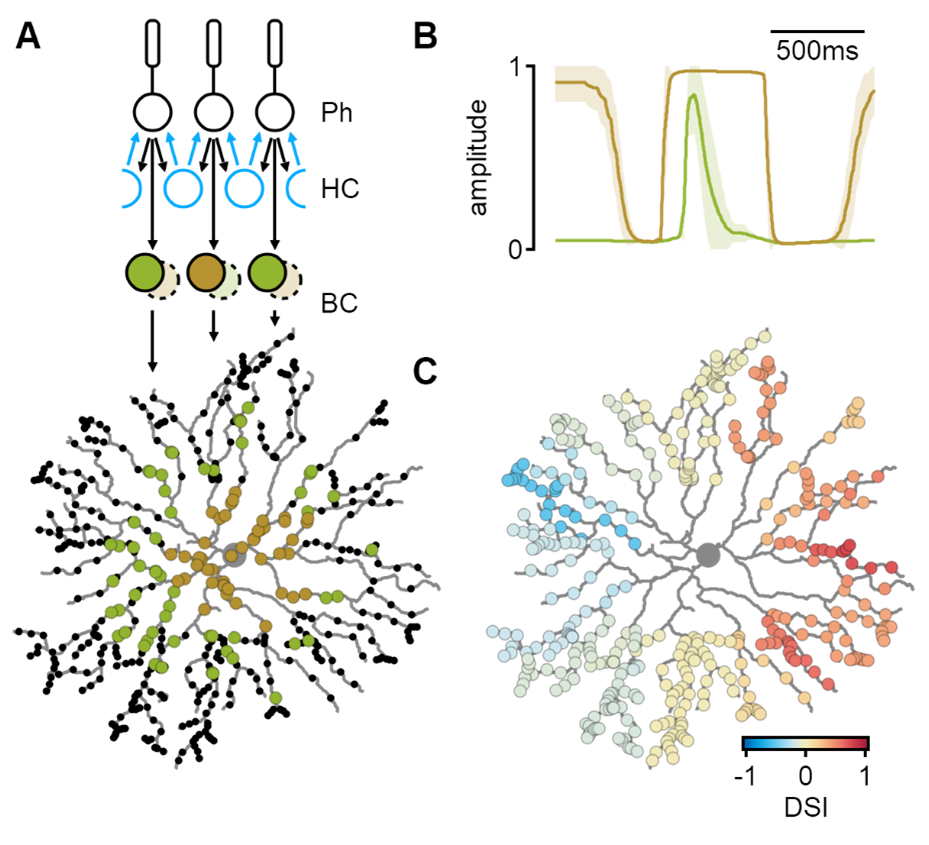
Simulated spatiotemporal integration in SACs. A) Bipolar cells (BCs) receive pre-processed visual input from the photoreceptor-horizontal cell (HC) network. Proximal and distal SAC regions are innervated by different bipolar cell types. B) Bipolar cell dynamics predicted by a machine-learning algorithm to produce the most considerable degree of directional tuning in SAC dendrites (C).

Novel circuit-level mechanisms of DS proposed by a systematic exploration in-silico. a) simulated responses in a network with spatially diverse synaptic weights could produce directional tuning. b) The optimal solution in a simulation with varying input kinetics was reminiscent of the established Reichardt detector model. c) Directional tuning could be driven by differences in presynaptic surround formulation.
Completed Projects
1. Representation of motion in retinal bipolar cells
We used AAV-introduced glutamate reporter iGluSnFR and two-photon microscopy to measure responses from bipolar cells to static flashes and moving objects. Bipolar cells represent an important class of retinal interneurons that transmit visual signals from photoreceptors to ganglion cells, which in turn, send processed information to other brain regions. We recorded from the entire bipolar population and identified several clusters of cells with distinct functional responses. Most interestingly, we found that responses to motion could not be predicted from the stationary waveform, indicative of a separate processing layer of moving objects, despite occurring in the same neuronal circuits.
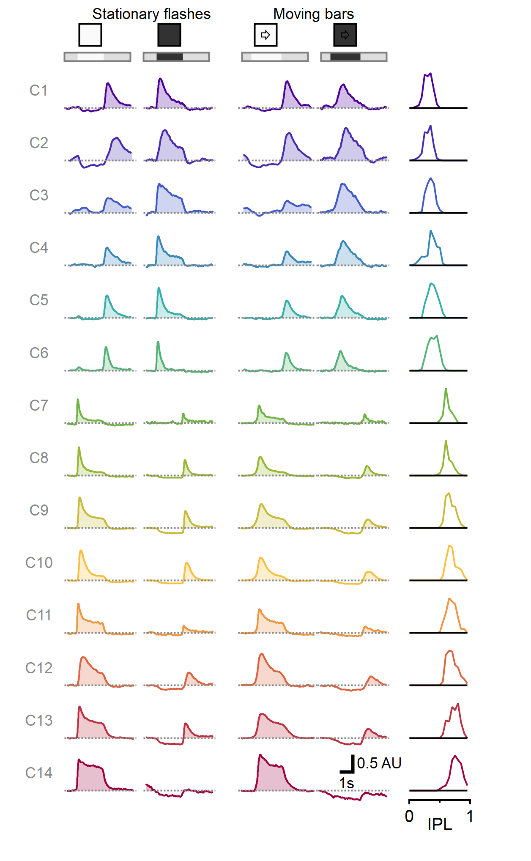
Multiplexed representation of static and moving objects in bipolar cells. Diversity of responses to stationary flashes and moving bars assorted across 1828 ROIs (278 scan fields in 67 animals), sorted by pixel depth distribution in the retina.
2. Center-surround receptive fields identify novel visual items
In a recently completed project, we proposed a functional role of circularly symmetric center-surround RFs as detectors of novel objects. This property is a logical but previously underappreciated consequence of the classic center-surround formulation. Continuously moving stimuli always enter the surround region first, driving the surround towards a more effective inhibition by the time the center is engaged. The priming effect on the surround is weaker or absent in suddenly appearing objects and objects that emerge from behind an occluder. Using pharmacological manipulations, we showed that novelty computation arises very early in the retinal processing, possibly at the level of photoreceptors.
Physiphysicals studies in humans provide evidence that the sudden appearance of new objects grabs attention reflexively; motion onset is less salient - but more noticeable than continuous motion. Our data propose that these computations are hard-wired in the retina and reflect the information content conveyed by the respective visual items.
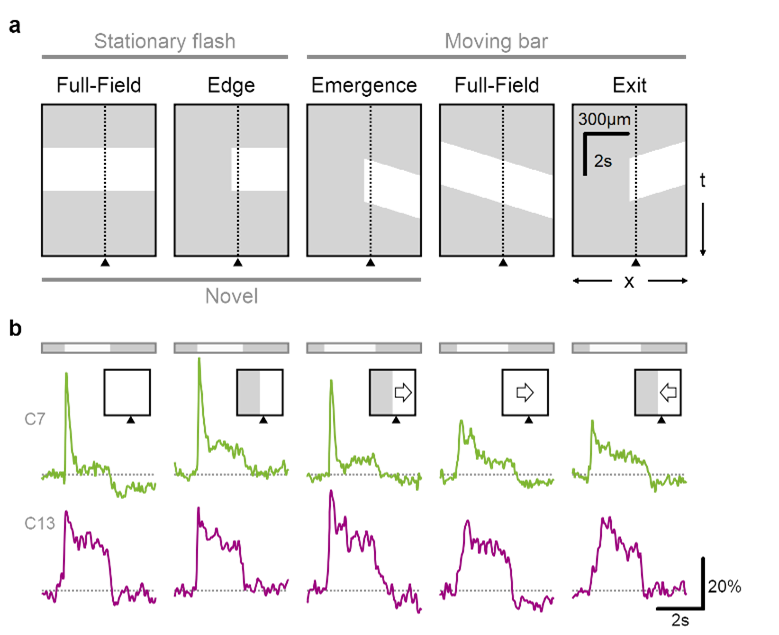
Glutamate release in the retina is sensitive to novel object appearance.
a Time-space plot indicating the position of the stimulus (white), presented either over the full extent of the display or masked by an occluder. Dotted line and triangle illustrate exemplar spatial receptive field center close to a mask-stimulus boundary. b Responses from two putative bipolar cells (green, transient; red, sustained dynamics) located near (<50 µm) a visual edge. Note the pronounced responses to emerging motion.
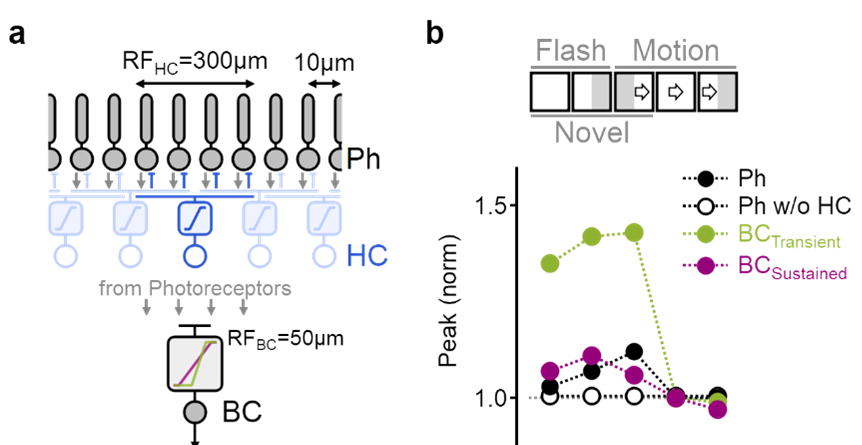
Detailed simulation of motion processing in the inner plexiform layer.
a) Photoreceptors (black) combined the visual input with feedback inhibition from horizontal cells (blue). Transient (green) and sustained (red) bipolar cells differed by the formulation of their dependency on the photoreceptor input. b) The model faithfully replicated experimentally observed bipolar cell responses to visual stimuli.
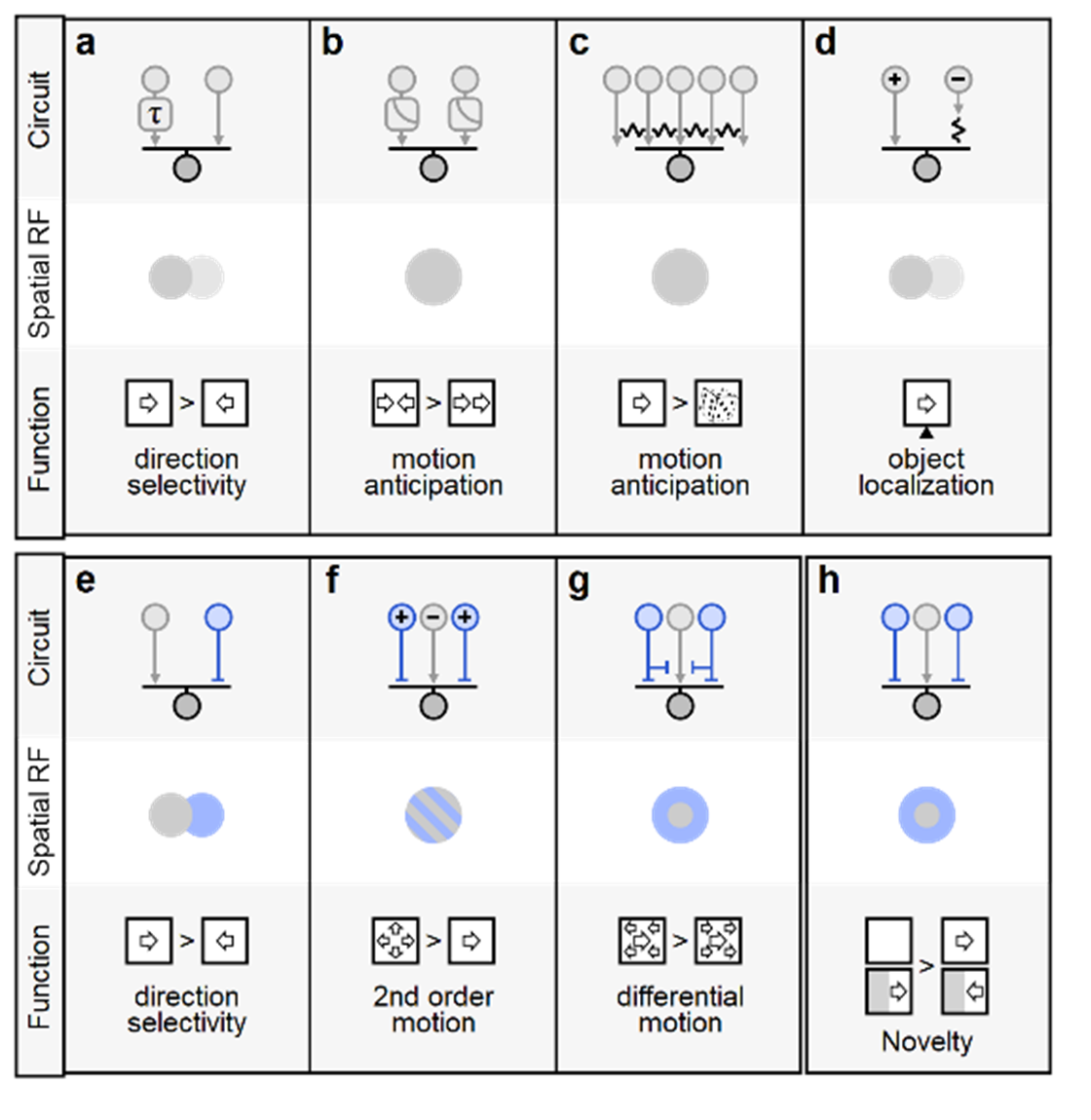
a Hassenstein-Reichardt correlator computes direction selectivity by integrating spatially separated inputs with distinct temporal dynamics (slower arm denoted by τ) b Contrast adaptation (gain-control) supports predictive encoding of moving objects, increased responsiveness to motion onset and detection of motion reversal. Electrical coupling among bipolar and ganglion cells can compensate for the lag in visual processing, increase sensitivity to correlated input vs. randomly shuffled stimuli (c), and assist in object localization (d). e-h Diverse contributions of inhibitory circuits (depicted in blue) to motion responses. A spatiotemporal offset between excitation and inhibition is the basis of the Barlow-Levick model of direction selectivity, found in many direction-selective ganglion cells (e). f Integration of presynaptic excitatory/inhibitory units with similar spatial receptive fields but reversed polarities (marked by '+' and '-'signs) facilitates detection of second-order and approaching motion. Center-surround organization in the segregation of local vs. global motion (g) and facilitate identification of novel items in the visual scene (h).
The multifaceted function of NMDA receptors
One of our main goals is to understand how NMDA receptors, a type of glutamatergic receptor, contributes to dendritic function. NMDA receptors are present in many brain regions and are a crucial component in neuronal excitability, synaptic plasticity and learning and memory. NMDA receptor dysfunction is a significant factor in the progression of many pathological conditions. In a way, NMDA receptors are the wild card of synaptic function – their computational role changes dramatically based on synaptic activation parameters and network architecture. For this reason, it is hard to prescribe a single operation or contribution to computations that NMDA receptors perform in-vivo. In the retina, NMDA receptors are expressed in many cell types; each of these cells is embedded in a different network infrastructure that facilitates extraction of specific features out of the visual scene. Thus, the retina presents an opportunity to study dendritic information processing in diverse computational tasks and provides a convenient system for theoretical comparison between different algorithms of neurocomputation. We combine use electrophysiology and two-photon imaging in genetically labeled cells to characterize cellular responses to the visual input and test the participation of NMDA receptors using pharmacological and genetic manipulations.
3. Unique noise capabilities of NMDA spikes
The brain operates surprisingly well despite the noisy nature of individual neurons. The central mechanism for noise mitigation in the nervous system is thought to involve averaging over multiple noise-corrupted inputs. Yet dendritic spikes, which are vital for many cortical computations, have miniscule integration compartment sizes and should be highly susceptible to noise. We performed biophysically accurate neuronal models to examine the performance of cells with dendritic spikes in noise environments. We found a new denoising approach mediated by focal dendritic spikes that depend on the extra thresholding step introduced by spike generation. It increases neuronal tolerance for a broad category of noise structures, some of which cannot be resolved well with averaging. This property of active dendrites compensates for compartment size constraints and expands the repertoire of conditions that can be processed by neuronal populations.
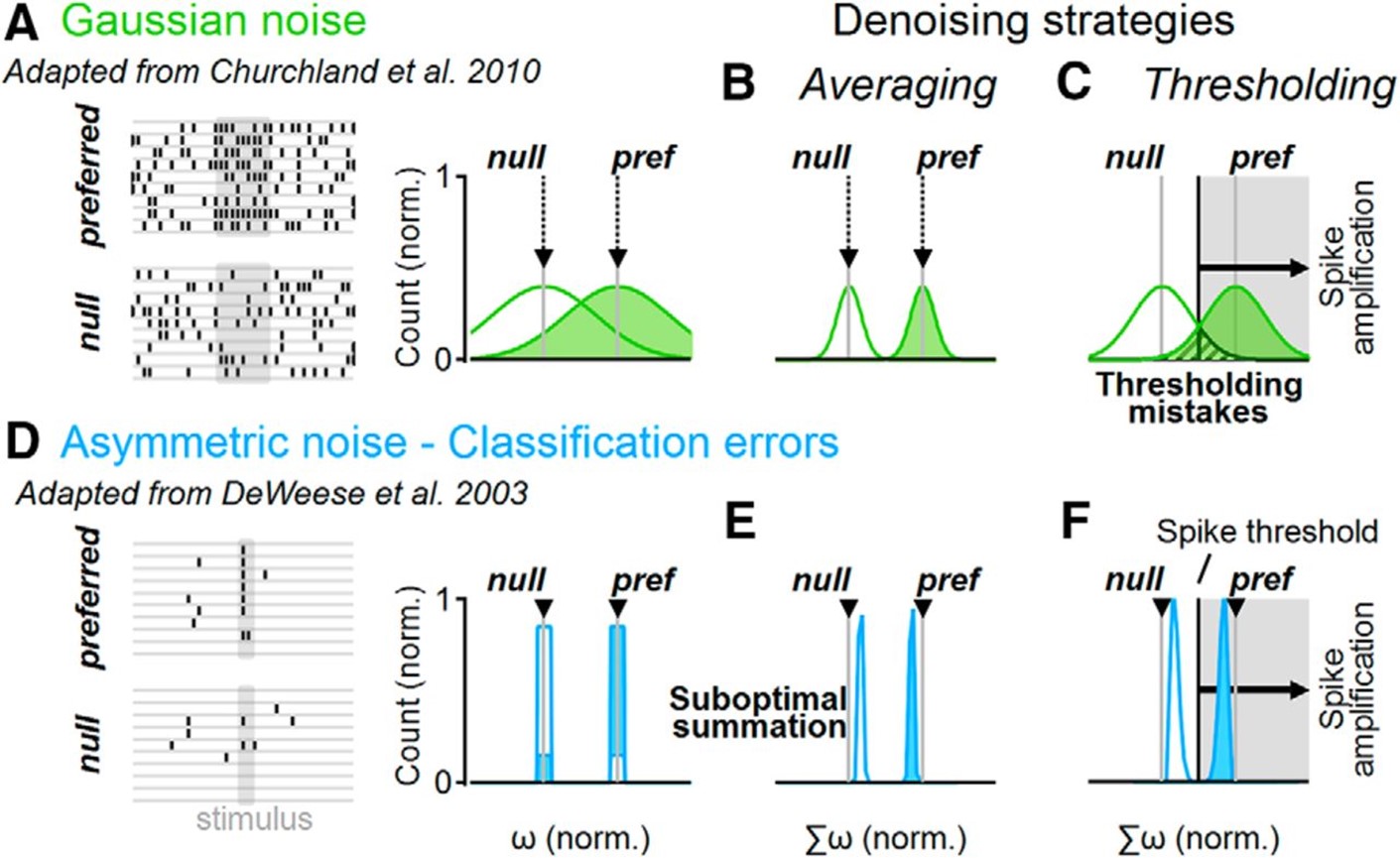
Distinct denoising operations in linear and active dendrites. A, Representative spike trains (left) in a simulated neuronal population that respond to a stimulus with normally distributed firing rates (right). The mean firing rates (gray) correspond to noise-free responses. B, Linear integration of a normally distributed population activity decreases the trial-to-trial variability of the postsynaptic cell. C, The effectiveness of denoising depends on the number of samples. D–F, As in A–C but for a simple asymmetric noise distribution of population responses, where preferred stimulation is signaled with a single spike. Some presynaptic cells produce “classification errors” if they spike to the null or do not produce a response to the preferred stimulations (D). E, Linear synaptic integration of an asymmetric presynaptic population shifts the mean output away from the noise-free population activity, diminishing the difference between stimuli representations (“suboptimal summation”). F, Spikes form a decision threshold that can help increase the separation between postsynaptic stimuli representations, compensating for the imperfect linear integration of asymmetric noises.
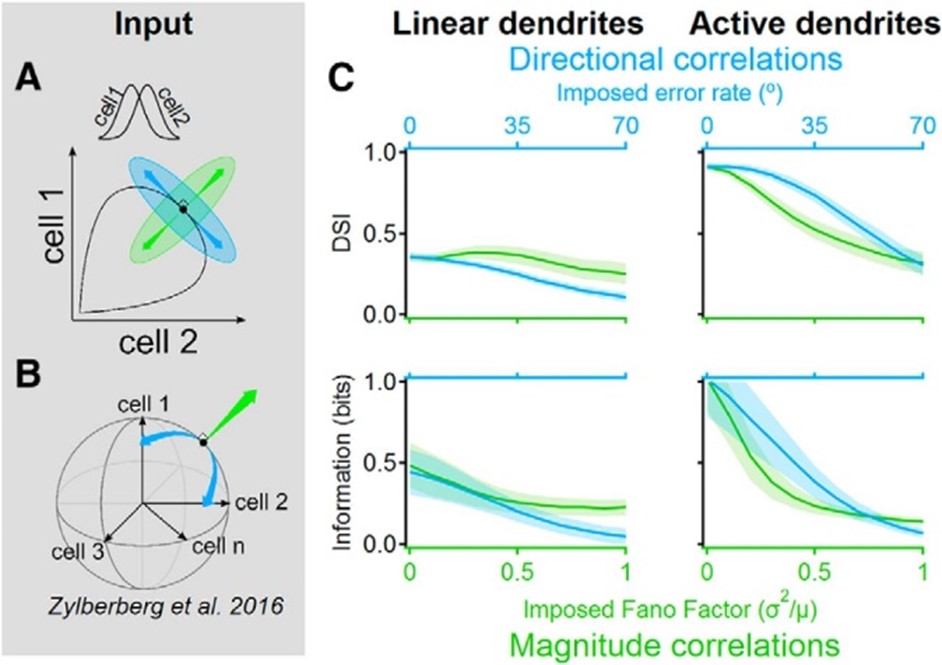
Signal-space noise correlations enhance the information capacity of active dendrites. Population noise can be described by a vector in the n-dimensional space where each dimension corresponds to the activity of a single presynaptic cell. The trial-to-trial correlation structure between neuronal responses determines the amount of information that can be represented by the population. A, Illustrated neural response space with two neurons with different directional tuning curves (inset). Black indicates the noise-free activity plotted for all possible stimulus angles; green indicates a “magnitude” noise structure that shifts the trial-to-trial response variability along a vector that points in the orthogonal direction from the population curve. Magnitude noise was shown to be well tolerated in linear dendrites. Blue indicates “directional” noise correlations, which translate the responses along a tangent to the curve, are known to diminish the information that can be extracted with linear integration. B, Similar to A but for n presynaptic cells. Directional correlations tend to vary the population activity along the signal space that mostly lies within the shell of a hypersphere. C, Postsynaptic direction selectivity index (DSI, top) and mutual information (bottom) versus imposed noise intensity in linear (left) and active (right) dendrites.
4. NMDA receptors enable multiplicative, and not additive, synaptic integration
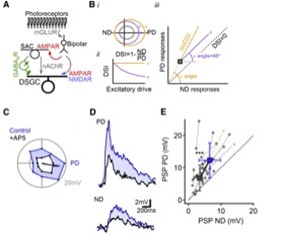
NMDARs Perform Multiplicative Scaling of Synaptic Inputs in DSGCs. (A) Retinal DS network schematic. For simplicity, only the ON pathway is shown. Visual information is conveyed from photoreceptors through bipolar cells to DSGCs by glutamatergic inputs that are not DS. DS is computed first in SACs, which provide inhibitory GABAergic and excitatory cholinergic drive to DSGCs. (B) Impact of additive (purple, +) versus multiplicative (yellow, ×) excitatory scaling of baseline responses (gray). (i) Additive scaling increases the responses by a constant in all directions of stimulation, whereas multiplication scales the responses in different directions proportionally. (ii) Multiplicative, but not additive, scaling preserves DS. (iii) On a PD versus ND plot, additive scaling follows a 45° angle line (right), whereas multiplicative scaling follows a line that connects the unscaled responses to the origin.(C) Polar plot of subthreshold responses in a DSGC to a bright bar moving across the retina in eight directions. (D) PD and ND responses from the same cell before (blue) and after NMDAR blockade by AP5 (black). (E) Postsynaptic reponses in control (blue) and AP5. Yellow (×) and purple (+) dashed lines indicate theoretical multiplicative and additive scaling of responses in AP5
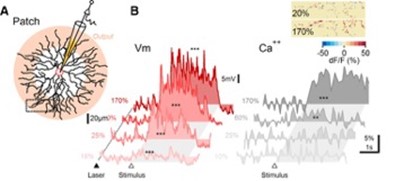
Membrane potential and dendritic calcium recording from SAC reveal different contrast dependence. (A) Schematic of experimental design. SACs were patched and filled with a calcium indicator. Pink shading indicates approximately the area of the dendrites from which GABA and ACh release occurs. Visual stimulation consisted of a bar moving in the centrifugal direction (ie, from the soma to the recorded dendritic location). (B) Representative somatic PSPs (left) and corresponding dendritic calcium transients 1 from the rectangular ROI in A (right). The dotted line and filled triangle mark the beginning of 2P laser scanning. The open triangle and the shaded area indicate the approximate entrance and the time interval over which the stimulus occupied the cell's receptive field. Inset, Heat map showing the spatial distribution of dendritic calcium signals from the dotted region in A for two stimulus contrast levels, color-coded by the peak dF/F values.
In our investigation, we utilize multiple and diverse techniques.
Patch clamp electrophysiology
We perform voltage clamp or current clamp recordings from various types of retinal cells. In some experiments, we use two-photon microscopy to target genetically labeled cells.
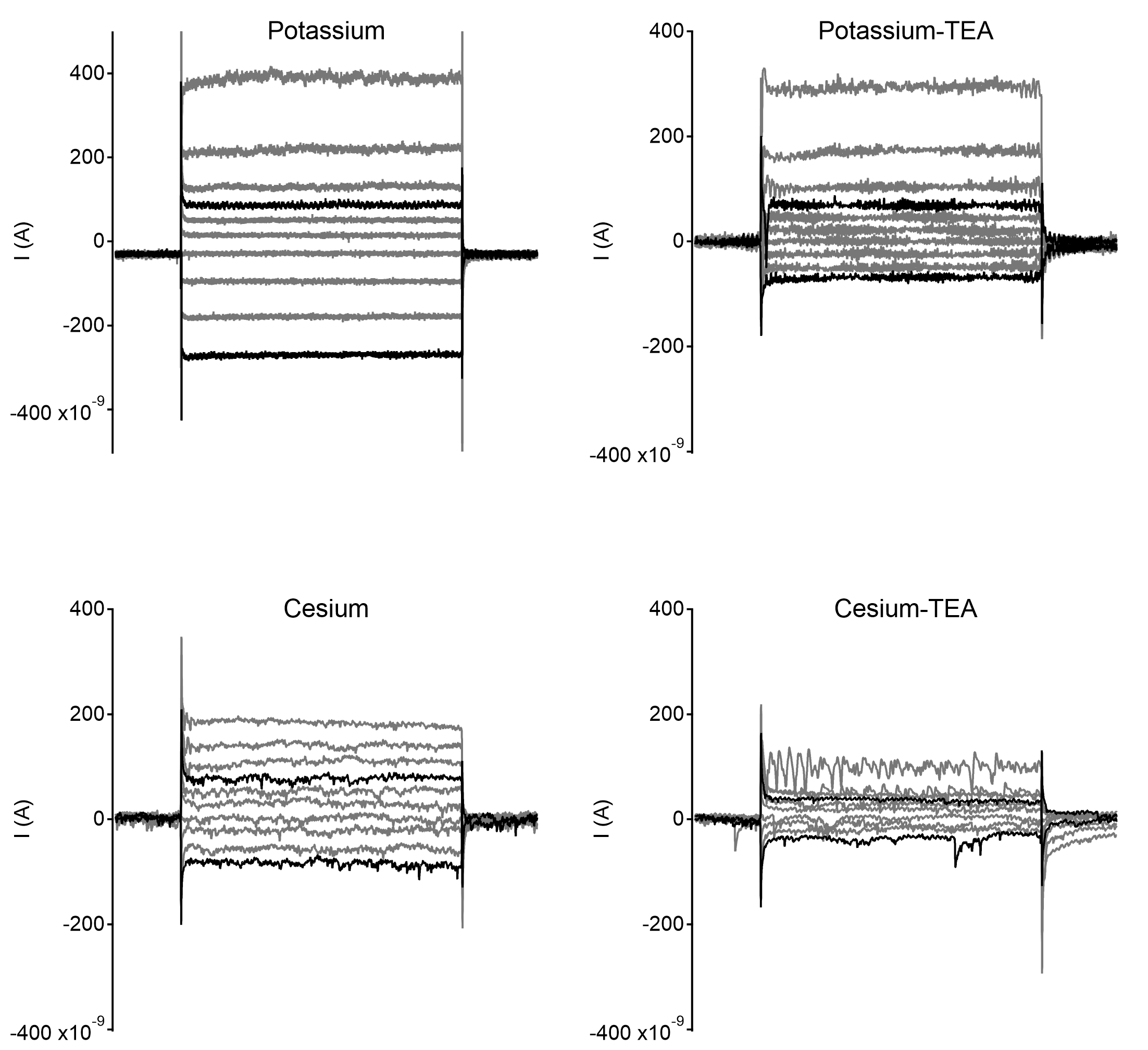
Example recordings from starburst amacrine cells with different internal solutions compositions. Each example shows currents elicited by voltage steps from -80mV, starting from -110mV up to -+20mV in steps of 10mVs. Traces with 30mV change in command potential in either polarity are highlighted in black.
Two-photon microscopy
Our uniquely designed two-photon microscope permits simultaneous recording of emitted fluorescence (for example, from calcium or glutamate sensors) and patterned visual stimulation.

Expressomics and transcriptomics
We use immunohistochemistry and in-situ hybridization with an RNAscope to obtain protein and gene expression information in specific retinal cells.
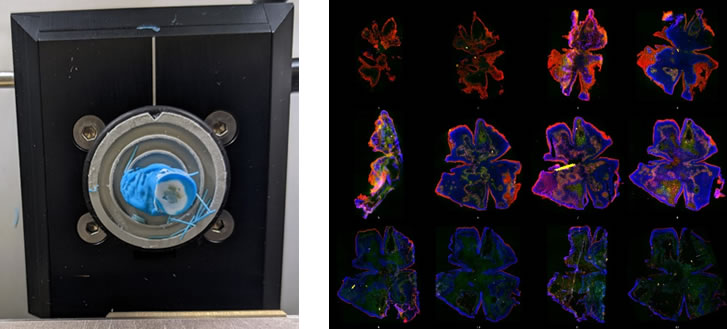
RNAscope analysis. Left, retina mounted in a cryo-stat block undergoing en-face sectioning. Right, a single retina sectioned into 15µm thick en face pieces from the outer to the inner retina stained to indicate different retinal layers.
In-vivo recording of visual reflexes
In collaboration with the Felsen lab, we apply a head-fixed paradigm and an immersive environment to record pupillary and optokinetic reflexes.
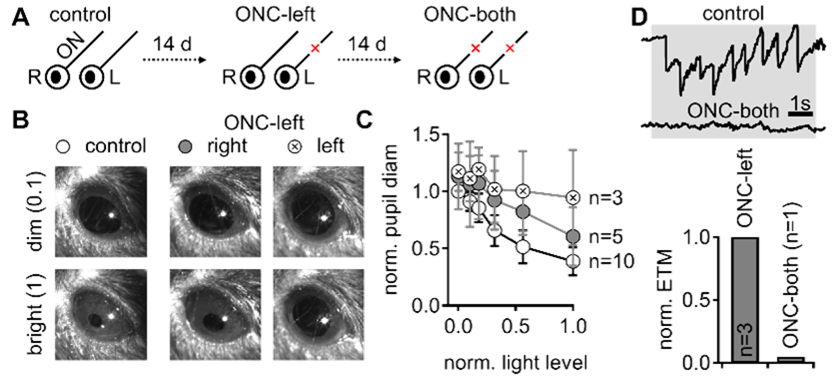
Example examination of reflexive visual behavior. A. Visual tests were performed prior to and following optic nerve crush. B. Representative direct pupillary light reflex in a head-fixed mouse. C. The fractional pupil constriction is absent following the left crush and reduced in the contralateral eye. D. the optokinetic reflex is diminished after bilateral nerve crush.
In-vivo electrophysiology
We record electroretinogram (ERG), pattern ERG and visually evoked potential (VEP). These techniques allow us to probe retinal function in general and visual responses from ganglion cells as well as the propagation of visual information to downstream regions.
In collaboration with Drs. Huang, Nagaraj and Nam we investigate the mechanisms of retinal damage and response to therapeutic intervention in traumatic brain injury (TBI) and glaucoma.
In-vivo transpupillary imaging
We develop single- and two-photon techniques to record calcium signals from retinal ganglion cells in response to visual stimulation in-vivo.

NEURON simulations
We develop biophysically-realistic models of cells and networks in the retina and the cortex. We emphasize dendritic computations and interactions between excitation and inhibition in specific visual tasks.
/group-photos/poleg-polsky-lab-2023.jpg?sfvrsn=33fd0bbb_2)
Alon Poleg-Polsky, Principal Investigator
Alon did his graduate work with Prof. Jackie Schiller (Technion, Israel) where he studied the contribution of dendritic NMDA receptors to the function of cortical pyramidal neurons. Alon showed that dendritic spikes form independent computational units within individual cells, enrich neuronal information processing and plasticity, create a unique communication code between cells and establish new rules for interactions between excitatory and inhibitory inputs in spiking dendrites.
He moved to Bethesda MD for his postdoctoral training in the lab of Jeffrey Diamond (NIH) to study visual processing in the retina. Alon helped to define novel network and synaptic mechanisms that ensure reliable responsivity in the direction selectivity (DS) circuit. He found that synaptic inputs, instead of summating together, interact multiplicatively, aided by activation of NMDA receptors. This action of NMDA receptors increases the gain of light-evoked responses, but unlike most electric amplifiers, which typically distort the signal (all too often a musical performance is ruined by a faulty amplifier), the unique mechanism of NMDA-mediated multiplication was shown to improve the fidelity of DSGC performance.
Elena Esch
Elena graduated with a BS in Neuroscience from Lafayette College in PA in 2018. During her time in college, she performed visual neuroscience research at New York University as a National Science Foundation Research Scholar and at the Massachusetts Institute of Technology as an Amgen Scholar. Following college, she did a two-year post-baccalaureate fellowship at the National Institute of Mental Health studying visual neuroscience and facial recognition processes. Elena then joined the University of Colorado’s Medical Scientist Training Program as an MD-PhD student in 2021. Clinically, Elena is interested in pursuing plastic and reconstructive surgery. For her PhD, Elena will be working on a multi-mentored translational eye transplantation project using a variety of models. Specifically, she will be assessing the neurological and functional outcomes of eye transplantation including the survival and function of the retina post-transplantation, optic nerve regeneration, and whole-eye preservation.
Bacmeister CM, Huang R, Osso LA, Thornton MA, Conant L, Chavez AR, Poleg-Polsky A, Hughes EG. Motor learning drives dynamic patterns of intermittent myelination on learning-activated axons. Nat Neurosci. 2022 Oct 25 (10):1300-1313.
Gaynes JA, Budoff SA, Grybko MJ, Hunt JB, Poleg-Polsky A. Classical center-surround receptive fields facilitate novel object detection in retinal bipolar cells. Nat Commun. 2022 Sep 26;13(1):5575.
Otor Y, Achvat S, Cermak N, Benisty H, Abboud M, Barak O, Schiller Y, Poleg-Polsky A*, Schiller J*. Dynamic compartmental computations in tuft dendrites of layer 5 neurons during motor behavior. Science. 2022 Apr 15;376(6590):267-275.
Poleg-Polsky A. Dendritic spikes expand the range of well-tolerated population noise structures. J Neuroscience, 2019 Nov 13;39(46):9173-9184.
Kumar A, Schiff O, Barkai E, Mel BW, Poleg-Polsky A*, Schiller J. NMDA spikes mediate amplification of inputs in the rat piriform cortex. Elife, 2018 Dec 21;7. pii: e38446. doi: 10.7554/eLife.38446.
Poleg-Polsky A, Ding H, Diamond JS. Functional Compartmentalization within Starburst Amacrine Cell Dendrites in the Retina. Cell Rep, 2018 Mar 13;22(11):2898-2908. doi: 10.1016/j.celrep.2018.02.064.
Ding H, Smith RG, Poleg-Polsky A, Diamond JS, Briggman KL. Species-specific wiring for direction selectivity in the mammalian retina. Nature, 2016, doi:10.1038/nature18609
Poleg-Polsky A, Diamond JS. Retinal circuitry balances contrast tuning of excitation and inhibition to enable reliable computation of direction selectivity. J Neuroscience, 2016, 36(21), 5861-76.
Poleg-Polsky A, Diamond JS. NMDA receptors multiplicatively scale visual signals in direction selective ganglion cells. Neuron, 2016, 89(6), 1277-90.
Poleg-Polsky A. Effects of Neural Morphology and Input Distribution on Synaptic Processing by Global and Focal NMDA-Spikes. PLoS One. 2015, 10(10), e0140254.
*co-corresponding author
We are looking for enthusiastic Postdoctoral Fellows with an interest in retinal function, neuronal signaling and/or neurological disorders. We welcome inquiries from graduate students regarding lab rotations. Short-term modeling and experimental projects are available and previous experience is not a requirement.