Kurt Beam, PhD
Professor
/kurt-beam----100-x-130.jpg?sfvrsn=e18861b9_4)
Department of Physiology and Biophysics
University of Colorado Anschutz
RC-1 North Tower, P18-7132
Mail Stop 8307
Tel (303) 724-4542
E-mail: [email protected]
Graduate Program Affiliations:
Curriculum Vitae:
CV (Kurt Beam, 2022)
(pdf)
230 KB
Shared Content Block:
Physiology & Biophysics Styles -- Borderless table for directories
My lab investigates intracellular calcium signals, which play essential roles including the activity-dependent regulation of gene expression in diverse cell types, muscle contraction, and neuronal plasticity. We use a variety of techniques including molecular biology, electrophysiology, biochemistry, and analysis of structure.
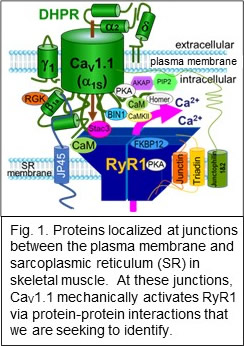
A major focus has been on the protein complex in skeletal muscle that links conformational changes of a plasma-membrane calcium channel (CaV1.1) to activation of Ca2+ release via RyR1 in the sarcoplasmic reticulum (Fig. 1).
As one approach for studying this process, we express cDNAs encoding key proteins in cultured muscle cells (myotubes). This animation illustrates GFP-CaV1.1 in myotubes null for endogenous CaV1.1: each green dot corresponds to ~100 calcium channels clustered in the plasma membrane.
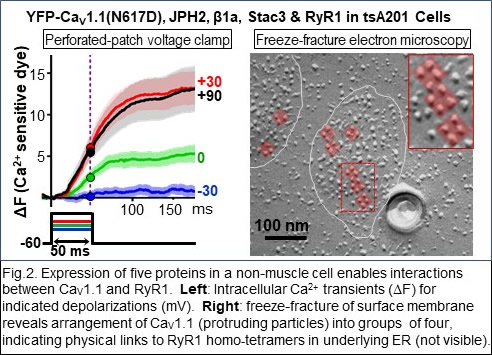
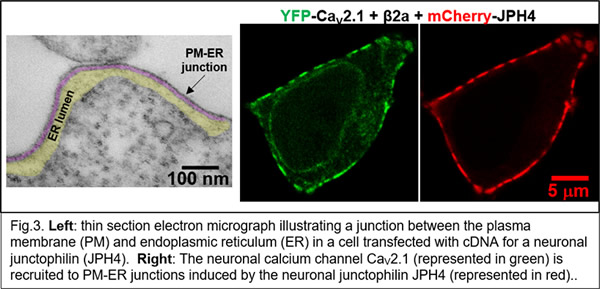
More recently (Ref. 4), we were able to identify a set of five proteins sufficient to reproduce the Ca2+ release process in skeletal muscle (Fig. 2) and are now using this system to identify the underlying molecular interactions. Isoforms of all five of these proteins are expressed in the nervous system, where much less is known about their biological roles. Our recent work has begun to examine how these proteins function in neurons (Fig. 3, Refs. 6-8).
Daniel Enders Professional Research Assistant |
/group-photos/beam-lab-2023.jpg?sfvrsn=68fb0bbb_2)
NIH Jacob Javits Neuroscience Investigator Award (1988-1995)
Thomas W. Smith Memorial Lecture, American Heart Association (1999)
K.S. Cole Award for Membrane Biophysics (2002)
Paul Horowicz Memorial Lecture, Rochester University (2005)
Elected to National Academy of Sciences (2012)
Totman Lecture, University of Vermont (2012)
University Distinguished Professor, University of Colorado (2014-present)
Szpyt, J, Lorenzon, N, Perez, CF, Norris, E, Allen, PD, Beam, KG, and Samsó, M. 3D Localization of the Alpha and Beta Subunits and of the II-III loop in the Skeletal Muscle L-type Ca2+ Channel. J. Biol. Chem. 287:43853-61, 2012.
Polster A, Perni S, Bichraoui H, and Beam KG. Stac adaptor proteins regulate trafficking and function of muscle and neuronal L-type Ca2+ channels. Proc Natl Acad Sci USA 112:602-606, 2015.
Polster A, Nelson BR, Olson EN, and Beam KG. Stac3 has a direct role in skeletal muscle-type excitation-contraction coupling which is disrupted by an inherited myopathy-causing mutation. Proc Natl Acad Sci U S A. 113:10986-10991, 2016.
Perni S, Lavorato M, and Beam KG. De novo reconstitution reveals the proteins required for skeletal muscle voltage-induced Ca2+ release. Proc Natl Acad Sci U S A. 52:13822-13827, 2017.
Polster A, Perni S, Filipova D, Bichraoui H, Ohrtman JD, Beam KG, and Papadopoulos S. Junctional trafficking and restoration of retrograde signaling by the cytoplasmic RyR1 domain. J. Gen. Physiol. 150:293-306, 2018.
Polster A, Dittmer PJ, Perni S, Bichraoui H, Sather WA, and Beam KG. Stac proteins suppress Ca2+-dependent inactivation of neuronal L-type Ca2+ channels. J. Neurosci. 38:9215-9227, 2018.
Perni S, and Beam KG. Neuronal junctophilins recruit specific CaV and RyR isoforms to ER-PM junctions and functionally alter CaV2.1 and CaV2.2. Elife 10:e64249, 2021.
Perni S, and Beam KG. Junctophilins 1, 2 and 3 all support voltage-induced Ca2+ release despite considerable divergence. J. Gen. Physiol., in press, 2022.