Linda F. van Dyk, PhD
Professor & Vice Chair
Department of Immunology & Microbiology
University of Colorado Anschutz School of Medicine

Linda van Dyk, Ph.D., earned her doctoral degree in Immunology from the University of Texas Southwestern in 1994, advised by Dr. Kathy Meek on studies of immunoglobulin gene rearrangement. She completed her postdoctoral research training at Washington University School of Medicine in 2001, advised by Dr. Steve Dowdy on studies of lymphocyte cell cycle regulation during cell activation and apoptosis, and then by Drs. Sam Speck and Skip Virgin on studies of a viral cyclin in gammaherpesvirus infection and host interactions. Dr. van Dyk joined the faculty of the University of Colorado School of Medicine in 2001.
Dr. van Dyk received the 2001 American Herpes Foundation Gertrude Elion Research Award, and was the recipient of the Burroughs Wellcome Fund Investigator Award in Pathogenesis of Infectious Disease. She is an associate editor for Frontiers of Immunology in Microbial Immunity and for Virology Journal in Herpesviruses. Dr. van Dyk has served as a member of the ASM Watkins fellowship review panel, and as an ad hoc member of NIH review panels including Special Emphasis, Virology A and Virology B study sections, and is currently a regular member of the AIDS, Opportunistic Infections and Cancer study section.
The van Dyk laboratory has been supported by the University of Colorado School of Medicine, the University of Colorado Cancer Center, the Cancer League of Colorado, the Colorado Foundation for AIDS Research, the University of Colorado Department of Immunology and Microbiology, the Burroughs Wellcome Foundation, the NIH National Cancer Institute and the NIH National Institute of Allergy and Infectious Diseases.
Dr. van Dyk received the 2001 American Herpes Foundation Gertrude Elion Research Award, and was the recipient of the Burroughs Wellcome Fund Investigator Award in Pathogenesis of Infectious Disease. She is an associate editor for Frontiers of Immunology in Microbial Immunity and for Virology Journal in Herpesviruses. Dr. van Dyk has served as a member of the ASM Watkins fellowship review panel, and as an ad hoc member of NIH review panels including Special Emphasis, Virology A and Virology B study sections, and is currently a regular member of the AIDS, Opportunistic Infections and Cancer study section.
The van Dyk laboratory has been supported by the University of Colorado School of Medicine, the University of Colorado Cancer Center, the Cancer League of Colorado, the Colorado Foundation for AIDS Research, the University of Colorado Department of Immunology and Microbiology, the Burroughs Wellcome Foundation, the NIH National Cancer Institute and the NIH National Institute of Allergy and Infectious Diseases.
The van Dyk lab studies the interactions between virus and host in health and disease. Specifically, our work focuses on gammaherpesviruses. It is said that herpesviruses are forever. This is because they are ancient, highly adapted to their hosts, and infect for life. Lifelong infection usually take the form of a highly quiescent, latent infection in healthy individuals. The latent infection is controlled by the host immune system, and therefore immunosuppression results in reactivation of lytic infection. A hallmark of the gammaherpesviruses is their ability to establish latent infection within the lymphoid system of the host. Gammaherpesviruses persist for life within cells of the immune system, the very system responsible for their clearance. This balance between the host immune system and viral latency is complex and is dependent on both host and viral genes. The gammaherpesviruses include the human pathogens Epstein Barr virus and Kaposi's sarcoma associated herpesvirus (KSHV), as well as murine gammaherpesvirus 68. Each of these viruses enter at mucosal surfaces and use B lymphocytes as a major reservoir of latency. The gammaherpesviruses are malignancies of B cells, as well as epithelial cells and endothelial cells.
 The focus of my lab is on gammaherpesvirus interactions, particularly on virus and host mechanisms to regulate latency and reactivation. Our studies make frequent use of the γHV68 murine model of gammaherpesviruses, a model that provides several benefits, including: genetic tractability of both virus and host, the ability to study virus/host interactions over the entire time course and tissues relevant to viral infection and disease. This model system provides broad insights into different gammaherpesvirus parameters of infection, with particular interactions between virus infection and host immunity underlying acute lethal pneumonia, splenic plasma cell lymphoma, vessel disease and perivascular inflammation, pulmonary lymphoma with secondary CNS lymphoma, and pulmonary hypertension/fibrosis.
The focus of my lab is on gammaherpesvirus interactions, particularly on virus and host mechanisms to regulate latency and reactivation. Our studies make frequent use of the γHV68 murine model of gammaherpesviruses, a model that provides several benefits, including: genetic tractability of both virus and host, the ability to study virus/host interactions over the entire time course and tissues relevant to viral infection and disease. This model system provides broad insights into different gammaherpesvirus parameters of infection, with particular interactions between virus infection and host immunity underlying acute lethal pneumonia, splenic plasma cell lymphoma, vessel disease and perivascular inflammation, pulmonary lymphoma with secondary CNS lymphoma, and pulmonary hypertension/fibrosis.
Gammaherpesvirus regulation by cyclins and cyclin homologs. Murine gammaherpesvirus 68 and KSHV both encode homologs of the host cyclins. Exogenous expression of the viral cyclins promotes cell cycle progression and leads to cellular transformation. The viral cyclins are required for reactivation from latent infection and for survival of autophagy during primary infection through distinct activities. The viral cyclins interact with host tumor suppressor proteins, particularly with the cyclin dependent kinase inhibitor, p18Ink4c. We investigate the mechanism of cyclin regulation in latency and reactivation using molecular biology, biochemistry, genomics and genetics.
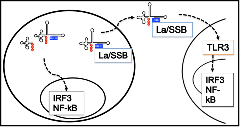 Non-coding RNA regulation of primary and chronic infection. We initiated studies viral non-coding RNAs by discovery that murine gammaherpesvirus 68 encodes miRNAs and found that these miRNAs were transcribed with viral tRNA-like genes with functions only now being discovered. We dissected the transcription and processing of these hybrid TMER genes and demonstrated that viral miRNAs are functional during infection, yet are atypical in their transcription and processing. We recently demonstrated that these small non-coding viral RNAs not only produce miRNAs, but also possess innate regulatory activity akin to the EBV EBER regulatory RNAs. The gammaherpesvirus small non-coding RNAs are therefore similar to the viral cyclin, in that they are multifunctional, with distinct molecular requirements in different phases of infection that we investigate using a wide range of techniques and models.
Non-coding RNA regulation of primary and chronic infection. We initiated studies viral non-coding RNAs by discovery that murine gammaherpesvirus 68 encodes miRNAs and found that these miRNAs were transcribed with viral tRNA-like genes with functions only now being discovered. We dissected the transcription and processing of these hybrid TMER genes and demonstrated that viral miRNAs are functional during infection, yet are atypical in their transcription and processing. We recently demonstrated that these small non-coding viral RNAs not only produce miRNAs, but also possess innate regulatory activity akin to the EBV EBER regulatory RNAs. The gammaherpesvirus small non-coding RNAs are therefore similar to the viral cyclin, in that they are multifunctional, with distinct molecular requirements in different phases of infection that we investigate using a wide range of techniques and models.
 The focus of my lab is on gammaherpesvirus interactions, particularly on virus and host mechanisms to regulate latency and reactivation. Our studies make frequent use of the γHV68 murine model of gammaherpesviruses, a model that provides several benefits, including: genetic tractability of both virus and host, the ability to study virus/host interactions over the entire time course and tissues relevant to viral infection and disease. This model system provides broad insights into different gammaherpesvirus parameters of infection, with particular interactions between virus infection and host immunity underlying acute lethal pneumonia, splenic plasma cell lymphoma, vessel disease and perivascular inflammation, pulmonary lymphoma with secondary CNS lymphoma, and pulmonary hypertension/fibrosis.
The focus of my lab is on gammaherpesvirus interactions, particularly on virus and host mechanisms to regulate latency and reactivation. Our studies make frequent use of the γHV68 murine model of gammaherpesviruses, a model that provides several benefits, including: genetic tractability of both virus and host, the ability to study virus/host interactions over the entire time course and tissues relevant to viral infection and disease. This model system provides broad insights into different gammaherpesvirus parameters of infection, with particular interactions between virus infection and host immunity underlying acute lethal pneumonia, splenic plasma cell lymphoma, vessel disease and perivascular inflammation, pulmonary lymphoma with secondary CNS lymphoma, and pulmonary hypertension/fibrosis.Gammaherpesvirus regulation by cyclins and cyclin homologs. Murine gammaherpesvirus 68 and KSHV both encode homologs of the host cyclins. Exogenous expression of the viral cyclins promotes cell cycle progression and leads to cellular transformation. The viral cyclins are required for reactivation from latent infection and for survival of autophagy during primary infection through distinct activities. The viral cyclins interact with host tumor suppressor proteins, particularly with the cyclin dependent kinase inhibitor, p18Ink4c. We investigate the mechanism of cyclin regulation in latency and reactivation using molecular biology, biochemistry, genomics and genetics.
 Non-coding RNA regulation of primary and chronic infection. We initiated studies viral non-coding RNAs by discovery that murine gammaherpesvirus 68 encodes miRNAs and found that these miRNAs were transcribed with viral tRNA-like genes with functions only now being discovered. We dissected the transcription and processing of these hybrid TMER genes and demonstrated that viral miRNAs are functional during infection, yet are atypical in their transcription and processing. We recently demonstrated that these small non-coding viral RNAs not only produce miRNAs, but also possess innate regulatory activity akin to the EBV EBER regulatory RNAs. The gammaherpesvirus small non-coding RNAs are therefore similar to the viral cyclin, in that they are multifunctional, with distinct molecular requirements in different phases of infection that we investigate using a wide range of techniques and models.
Non-coding RNA regulation of primary and chronic infection. We initiated studies viral non-coding RNAs by discovery that murine gammaherpesvirus 68 encodes miRNAs and found that these miRNAs were transcribed with viral tRNA-like genes with functions only now being discovered. We dissected the transcription and processing of these hybrid TMER genes and demonstrated that viral miRNAs are functional during infection, yet are atypical in their transcription and processing. We recently demonstrated that these small non-coding viral RNAs not only produce miRNAs, but also possess innate regulatory activity akin to the EBV EBER regulatory RNAs. The gammaherpesvirus small non-coding RNAs are therefore similar to the viral cyclin, in that they are multifunctional, with distinct molecular requirements in different phases of infection that we investigate using a wide range of techniques and models.
Under construction
Immunology Microbiology (SOM)
CU Anschutz
Research I North
12800 East 19th Avenue
Mail Stop 8333
Aurora, CO 80045
303-724-4224
School of Medicine
General
Students
CMS Login