We are interested in understanding how T cell interactions with antigen presenting cells at the disease site can result in either T cell activation or tolerance. Our goals are to understand how the immune system dynamically regulates immune responses through cellular interactions and environmental cues, with the objective of developing therapeutic interventions to disrupt pathogenic cellular interactions that promote autoimmunity.
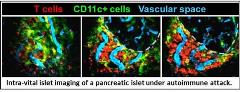
Type 1 diabetes is an autoimmune disease in which the immune system attacks the insulin-producing beta cells of the pancreatic islets of Langerhans. Autoreactive islet-specific T cells are one of the primary mediators of beta cell destruction. We are working in moue models of type 1 diabetes to understand the cues at the disease site that promote T cell pathogenesis. More specifically, we are interested in elucidating how cellular interactions between T cells and APCs in the islets modulate T cell pathogenesis in type 1 diabetes. To do so we have developed a novel imaging methods to analyze live pancreatic islets via 2-photon microscopy that allows us to analyze immune cell motility and interactions within the islets, trafficking, and islet infiltration during type 1 diabetes (see video).
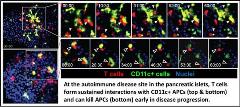
Determining the outcome of the cellular interactions we analyze is an important next step in gleaning functional data from 2-photon microscopy. To that end, we have also developed methods to image biosensors that can dynamically read out cellular signaling or effector function acquisition in vivo. This allows us to directly analyze signaling events and modulation of cytokine production that occur during cellular interactions within their native tissue environment. Importantly, this will enable us to study the events and functional outcomes in the pancreatic islets that lead to pathogenic T cell activation or protective tolerance in real-time with the long term goal of therapeutically blocking the events or cellular interactions that induce T cell pathogenesis.

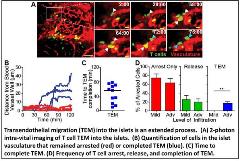
We are also interested in understanding the mechanisms of T cell extravasation into the pancreatic islets. Using a novel method for intra-vital imaging of the pancreas, we have been able to directly observe and analyze the process of transendothelial migration (TEM) into the islets for the first time (see video). We have found that this process is much slower and more restrictive than TEM into the lymph nodes. Our goals are to identify determinants of TEM into the pancreatic islets and preferentially them to prevent TEM into the islets without preventing normal T cell trafficking throughout the body. In collaboration with the Jacobelli lab, we are testing whether targeting cytoskeletal effectors of actin polymerization will accomplish that goal.
Click here for a list of Dr. Friedman's current publications.

