Using the Cytometry Lab
The ImmunoMicro Flow Cytometry Shared Resource Lab is in a secured area of the RC-1 North. An Anschutz Medical Campus ID with proper authorization is needed to access the portion of the building where the lab is located. Contact the SRL Manager if you do not have the required authorization.
To gain access to the lab:
- Sign up for an iLab account. iLab is the web application that manages scheduling and billing of flow cytometer usage. Detailed instructions for how to set-up an iLab account can be found here
- Have your Principal Investigator or lab manager associate a speedtype with your iLab account. Speedtypes are numerical codes that are used to pay for your instrument usage.
- Instructions for adding a new lab member can be found here.
- Instructions for adding funding numbers/speedtypes can be found here.
- E-mail SRL staff to schedule training. Depending on the amount of previous flow cytometry experience, analyzer training consists of 1-3 [60-120] min training sessions. Cell sorting requires demonstration of competence on the LSRFortessa, followed by additional training on the cell sorter.
- 1st session: review lab procedures, review instrument, and set-up experiment with beads that I provide (for inexperienced users) or cells provided by user (for experienced flow cytometrists). If experienced users can demonstrate competence operating the instrument, then no additional training is necessary.
- 2nd session: review lab procedures and run samples you provide. This is an opportunity to set-up templates and optimize the instrument for the experiments you expect to be doing. If the user can demonstrate competence operating the instrument, then no additional training is necessary.
- Optional 3rd session: repeat 2nd session activities until competence demonstrated.
Once training is completed satisfactorily, SRL staff will grant you ID access to the Cytometry Lab and independent usage status in the iLab scheduler.
- All institutions receiving grant or contract awards from NIH are required to follow current health and safety guidelines. Please review the University of Colorado | Anschutz Medical Campus statement on Flow Cytometry and Biosafety updated January 2020.
All human samples must be fixed before analysis.
All animal samples bearing agents transmissible to humans must be fixed before analysis.
A biosafety risk assessment must be performed before the start of ALL cell sorting projects and ALL analytical flow cytometer runs with UNFIXED human cells or potentially infectious material. Please e-mail the SRL staff to request a link to the proper forms. PLAN AHEAD. Depending on preexisting service commitments and the complexity of the hazards involved, performing a risk assessment and developing and implementing a risk mitigation plan can take several days.
- Check your samples. If there are visible particulates, the samples must be filtered before being placed on the cytometers. All samples to be placed on the sorters must be filtered just prior to the sort. Filters are available in the Flow Lab.
- You are responsible for keeping users of the Flow Lab safe. You are required to inform the SRL via the iLab appointment form if your samples contain a biohazard.
- You are responsible for cleaning the area around the cytometer after your sample run.
- You are responsible for cleaning the cytometer after your sample run. Specific cleaning procedures are posted at each instrument.
- You are the primary caretaker of your data. Transfer your data from the cytometer to the server immediately following your sample run.
Are there any specific biosafety considerations for cell sorts?
Cell sorts have specific biosafety requirements. Flow cytometry and cell sorting should be included in your IBC Authorization. A biosafety risk assessment must be performed before the start of ALL cell sorting projects. At least 10 business days before the scheduled sort, please complete a CELL SORT BIOSAFETY RISK ASSESSMENT REQUEST.
What types of cells can I sort?
Any type of cells, which can be easily kept in single cell suspension, can theoretically be sorted. Cells that easily clump, agglutinate, or settle out of suspension can be problematic causing clogging of the nozzle and disturbance of proper droplet formation. Primary human samples or samples infected with BSL2 pathogens require special handling and engineering precautions.
Can the SRL perform sterile sorts?
While absolutely sterile sorting is not technically possible, sorts can be done in an aseptic manner with no resulting contamination.
How will cell sorting affect my cells’ viability?
The effects of sorting on cells depends on several factors:
- Cell Type: Some cell types are more fragile than others. Any type of cell that has structures on the outside of the cell membrane is more likely to be damaged during the sorting process.
- Pre-sort Cell Status: Sorting is stressful to cells. Cells that are stressed pre-sort (through activation or drug treatment, etc.) may be more susceptible to the effects of sorting.
- Speed (Pressure) of Sort: The faster the sort, the higher the pressure. Some cells (i.e., DCs) do not do well under high pressure conditions. Faster is not necessarily better.
- Buffers: Most cells will not tolerate significant mixing of buffers. Carbonate cell culture buffers when mixed with phosphate buffers (sorting fluid) can cause precipitates to form on cell membranes compromising viability and/or function.
How should I prepare my cells for sorting?
Before sorting, the cell isolation procedure should be optimized to provide a single cell suspension of live cells with very little contaminating debris (dead cells, DNA, anything sticky). Cell Viability, autofluorescence and cell aggregation may all affect the quality of cell sorting experiments. Good sample preparation is crucial and will result in better sort purity, yield and post-sort cell function and viability.
Cell sorting requires cells in a single cell suspension. Cell clumping can be a problem with adherent cells, activated cells, or samples with a high percentage of dead cells. If the cells are clumped, they cause several problems:
- A large clump will clog the cell sorter which causes a delay and may contaminate the collection tubes.
- Clumped cells will also reduce the sort yield because they will be excluded from the singlet gate.
- Aggregated cells cause more coincidence (or software) aborts.
To minimize clumping, avoid keeping cells at unnecessarily high concentration. Keep the cell suspension at 1-10 million/mL during processing, depending on cell type.
Resuspend cells for sorting in a phenol red-free pre-sort buffer:
- Basic Pre-sort Buffer Recipe and recommendations for optimization
- Commercially available pre-sort buffers
Count your cells after staining and before sorting. Viability should be greater than 90%.
Polypropylene (PP) tubes are recommended for both sample and collection tubes. Cells are less likely to adhere to these tubes than to polystyrene (PS) tubes.
Immediately before sorting (at the sorter for cells prone to clumping), filter your samples with a 40-50 micron mesh filter (40-μm cell strainer cap; BD Falcon Cat #352340) to remove as many clumps as possible. Keep in mind that filtration of your cells will decrease your cell number, so make sure you begin with adequate cell numbers.
What concentration should my samples be at for sorting?
The ideal cell concentration for most sorting material is 10-20x106 cells/ml in a minimum of 0.5 ml pre-sort buffer. For cells prone to clumping, use a lower concentration. Cells should be counted after all sample staining and other preparation as it is not uncommon to lose up to 50% of cells during the staining process.
How many cells do I need to bring?
Factors to consider when determining how much starting material you need to bring to the sort:
- What is the typical viability of your sample prep? Remember to include a viability dye in your staining. Dead cells are more likely to bind reagents non-specifically and to form doublets.
- What is the quality of your single cell suspension? Are your cells sticky? Remember, only singlets are sorted. Doublets are directed to waste.
- What is the frequency of your population? Plan on a final recovery of about 75% of the starting number of desired cells. Is this a rare event sort? Rare event sorts (1% or less) can produce a lower recovery, as low as 50%.
- If possible, provide enough starting material to allow for a purity check of the product at the end of the run.
For example, if you want to recover 2 x 106 GFP+ cells from the sort and 90% of your cells are alive, 90% of your live cells are singlets, and 30% of your live singlet cells are GFP+, then:
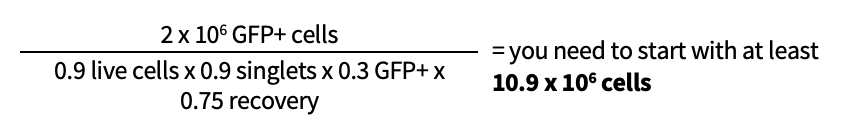
It is always best to calculate the number of input cells based on the worst-case scenario of a 50% yield rate so you will be sure to have an ample number of cells to start.
What is yield and why is it only 75-90%?
Yield is defined as “the {[number of cells in the collection tube] X [the % purity]} /{ [the number of cells you started with] X [% of the target population]}. There are several factors that affect the actual yield of the sorted sample:
- Electronic Aborts: Cells which arrive in the laser beam too close to one another; with each electronic abort, more than one cell is thrown away and not processed.
- Sort Conflicts: When a target and non-target cell occur within the same or overlapping sort envelopes; both target and non-target cells are not sorted.
- Loss of target cells due to cell death (pre- and post-sort) or adherence to tube walls.
Increasing sample rate (#cells/second analyzed) and the presence of cell aggregates can increase these losses and ultimately reduce your yield.
How long does an average sort take?
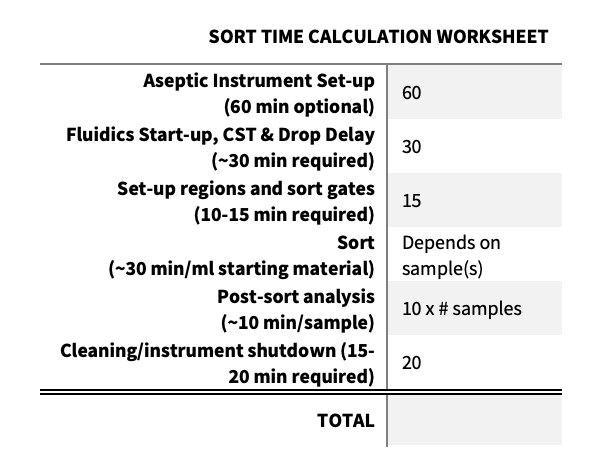
This is dependent on how many cells you need to recover and the % of target cells in the original sample. If you are sorting for a rare population of cells, it will generally take much longer than if you are sorting a population that is 30-50% of your original sample. 2.0 x 107 cells can take anywhere from 1.0-2.0 hours, depending on the pressure, quality of sample, and size of the cells (larger cells require a larger nozzle which necessitates lower pressures). Set up time for a sort takes about 60-90 minutes, 10-15 minutes to establish regions and sort gates, 10-15 minutes for post sort analysis, and 15-20 minutes for post-sort cleaning and instrument shutdown. The minimum cell sorter reservation is 2 hours.
How many populations can I sort simultaneously?
Up to four cell populations can be sorted simultaneously (microfuge or 12x 75 mm 5ml tubes). The remainder of cells are passed to waste. Each population can be identified with multiple parameters, i.e. multiple fluorescent probes, size and internal complexity. It is often useful to sort a negative (not the cells of interest) and positive populations (cells of interest) to have an internal control for whatever assay is performed on the sorted sample. The software limits gating strategies to a maximum of an eight-gate cascade.
What size collection tubes or plates are compatible with the cell sorter?
Sort collection tubes should be polypropylene (PP). Cells are less likely to adhere to PP tubes than to polystyrene (PS) tubes. The following standard formats are compatible with the cell sorter:
- Tubes
- Microfuge tubes (1-4 populations)
- 12x75mm 5mL tubes (1-4 populations)
- 15mL tubes (1-2 populations)
- Multi-well plates (1 population)
- 6, 12, 24, 48, 96, or 384 wells
- 6, 12, 24, 48, 96, or 384 wells
How should collection tubes/plates be prepared?
- Polypropylene collection tubes should be used to minimize the accumulation of an electrical charge on the tube.
- Collection tubes should be coated with proteins to avoid the sorted cells sticking to the tube wall causing reduced recovery and viability. This can be done by filling the tubes with 10% FCS (fetal calf serum) 30 minutes before sorting or incubating overnight at 4°C with 10% BSA (bovine serum albumin). Collection containers should never be dry but should be prefilled with a small volume of collection media optimized for the cells of interest. This prevents the dehydration of the sorted cells and keeps the cells under optimal conditions to ensure their viability.
- Collection media is diluted by the droplets being sorted. Use a concentration of collection media/FBS that will be diluted to the desired concentration by the volume of the sort.
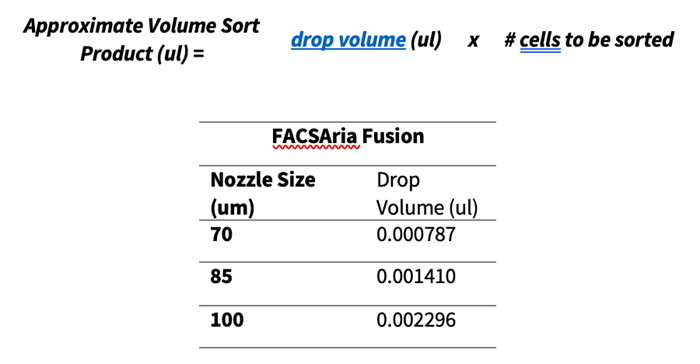
- Before placing the tube in the collection device, coat the walls with the collection media.
How should I treat my sorted sample to insure the best recovery and viability?
Sorting can be traumatic for cells. The cells should be spun down as soon as possible following the sort, re-suspended in their usual medium at their preferred concentration, and cultured under their usual conditions. Keep in mind that the condition of the cells prior to the sort will greatly impact their viability in the end. The healthier they are going into the sort the better the viability post-sort.
How do I schedule a cell sort?
At least 10 business days, before the scheduled sort please complete the following links.
Planning your Cell Sorting Experiment
Your experimental protocol should include the following elements:
- A cell isolation procedure that has been optimized to provide a single cell suspension of live cells with very little contaminating debris (dead cells, DNA, anything sticky). DNAse or EDTA can be added to cell preps that tend to clump.
- A viability dye. Dead cells are sticky and may non-specifically bind antibodies resulting in the sorting of false positives. Dead cells will be gated out of the sort if a viability dye is included in the staining panel.
- Fc Receptor Block to reduce the non-specific binding of antibodies to cells that do not bear the target.
- An optimized staining panel.
- Appropriate experimental controls. For example:
- Positive and negative controls
- Stimulated/treated and unstimulated/untreated controls
- Mock transfected controls
You MUST provide the following controls:
- Unstained cells
- Single color compensation controls for each fluorophore.
- A viability control. Viability controls can be generated by heat killing an aliquot of cells and mixing them with an equivalent number of live cells. Viability controls must have a live and a dead population.
- Gating controls to establish appropriate sort gates:
- A positive gating control.
- Fluorescence Minus One (FMO) controls. The FMO control ensures that the any spread of the fluorochromes into the channel of interest is properly identified.
Contact:
Tinalyn Kupfer, PhD, SCYM(ASCP)CM
Manager, Flow Cytometry Shared Resource Laboratory
E-mail:
tinalyn.kupfer@cuanschutz.edu
Address:
ImmunoMicro Flow Cytometry Shared Resource Lab
University of Colorado Anschutz Medical Campus
Mail Stop 8333 AMC
Research Complex 1 North | Room P18-8207
12800 E 19th Avenue,
Aurora, CO 80045
Phone: 303.724.8972
Immunology Microbiology (SOM)
CU Anschutz
Research I North
12800 East 19th Avenue
Mail Stop 8333
Aurora, CO 80045
303-724-4224
School of Medicine
General
Students
CMS Login