
Data and Safety Monitoring Committee
The DSMC reports to the Associate Director (AD) for Clinical Research who, in turn, reports to the Director of the CU Cancer Center. The DSMC ensures that research data generated by CU Cancer Center investigators are of high quality, reliable, and verifiable. Additionally, the DSMC is responsible for ensuring the safety of clinical trial participants. The DSMC provides oversight through:
- Review of DSM progress reports for drug/device interventional IITs.
- Conduct of internal audits.
- Ongoing monitoring of all Serious Adverse Events (SAEs) and Unanticipated Problems (UAPs) for all studies.
- Supervision of independent DSMBs for CU Cancer Center investigator-initiated large randomized trials that otherwise do not have an external DSMB assigned.
The DSMC identifies, develops and offers a variety of instructional activities to Investigators and their research personnel to ensure compliance with clinical trial management standards.
Collaboration
The DSMC is a requirement of the National Cancer Institute (NCI) and is intended to function as a helpful support team. The DSMC collaborates with PRMS, COMIRB, CU Denver Associate Vice Chancellor for Compliance, the School of Medicine Clinical Trials Advisory Committee, University of Colorado Hospital Research Review Committee and others to make the clinical trials process more efficient and accurate. For further information on the DSMC, please email [email protected].
Protocol Development Support Services
In support of investigator-initiated research at the CU Cancer Center, the DSMC has developed template language for the data and safety monitoring component required within all interventional investigator-initiated trial protocols. These templates are available in the table below and correspond with the trial types listed under the "Trial Description" column. The following steps need to be taken in order to obtain and use these templates:
- Identify the trial type of the protocol in development in the table below that best matches one of the categories under the "Trial Description" column.
- Click on the description as it is a link to the template language.
- Review the template language.
- Make any modifications (as needed) to this language based on the protocol’s needs or risks.
- Insert the data and safety monitoring plan language within the protocol.
- Submit the final protocol to the Protocol Review & Monitoring System (PRMS) through the PRMS protocol submission process.
Please note that the template language is meant to serve as a guide when developing the data and safety monitoring component of the investigator-initiated protocol, and is NOT meant to serve as a substitute for the protocol. Investigators are encouraged to review the template language and make modifications as needed prior to inserting the language into their protocol. If there are any questions regarding the template language or the development of a protocol-specific data and safety monitoring plan please contact the DSMC at [email protected]
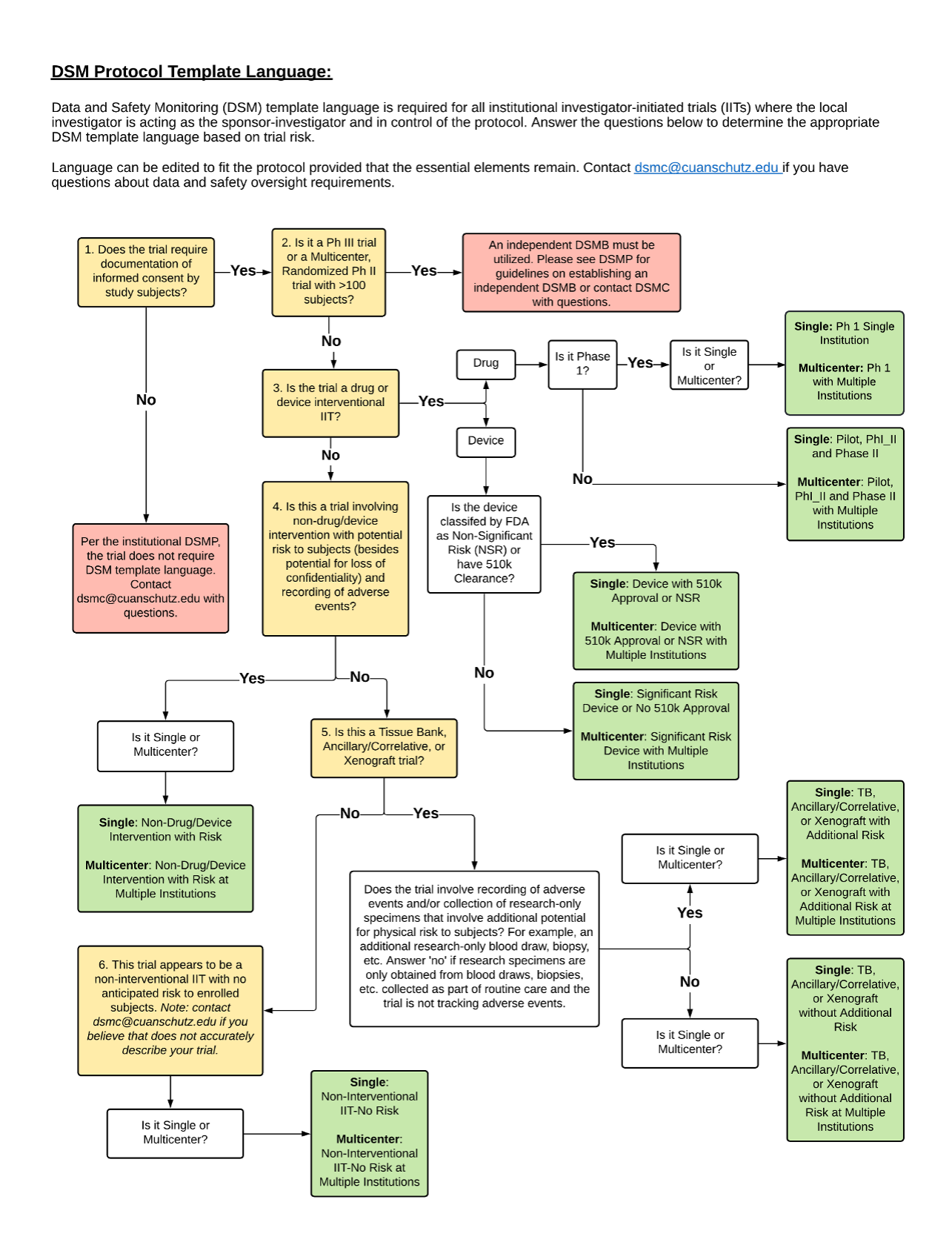
Definitions:
Interventional: Individuals are assigned prospectively by an investigator based on a protocol to receive specific interventions. The participants may receive diagnostic, treatment, behavioral, or other types of interventions. The assignment of the intervention may or may not be random. The participants are followed, and biomedical and/or health outcomes are assessed.
Observational: Studies that focus on cancer patients and healthy populations and involve no prospective intervention or alteration in the status of the participants. Biomedical and/or health outcome(s) are assessed in pre-defined groups of participants. The participants in the study may receive diagnostic, therapeutic, or other interventions, but the investigator of the observational study is not responsible for assigning specific interventions to the participants of the study.
Ancillary: Studies that are stimulated by, but are not a required part of, a main clinical trial/study, and that utilize patient or other resources of the main trial/study to generate information relevant to it. Ancillary studies must be linked to an active clinical research study and should include only patients accrued to that clinical research study. Only applicable for studies that can be linked to individual patient or participant data.
Correlative: Laboratory-based studies using specimens to assess cancer risk, clinical outcomes, response to therapies, etc. Only applicable for studies that can be linked to individual patient or participant data.
DSMC Template Language
Institutional Data Safety Monitoring Plan
The University of Colorado Cancer Center (CU Cancer Center) is dedicated to uniting our community to overcome cancer through innovation, discovery, prevention, early detection, multidisciplinary care, and education. To fulfill this mission, the CU Cancer Center incorporates the expertise of cancer specialists, state-of-the-art technology, and careful evaluation in the conduct of its clinical trials. The CU Cancer Center is committed to ensuring the safety of clinical trial participants and to maintaining data accuracy and protocol compliance.
The CU Cancer Center Data and Safety Monitoring (DSM) Plan has been developed to coordinate and provide oversight for the data and safety monitoring of all CU Cancer Center clinical trials, including trials conducted at both internal and external sites subject to CU Cancer Center oversight. This DSM plan is consistent with the National Institutes of Health (NIH) Policy for Data and Safety Monitoring (June 10, 1998) and Further Guidance on Data and Safety Monitoring for Phase I and Phase II Trials (June 5, 2000) as well as the National Cancer Institute (NCI) Data Safety Monitoring Guidelines (Approved 9/30/2014) and Data and Safety Monitoring of NCIH-Funded Clinical Research Policy (Reviewed 9/30/2014).
Read the Whole Report
Data Safety Monitoring (DSM) Progress Reports for Investigator-Initiated Trials
Principal Investigators of investigator-initiated drug/device interventional clinical trials (IITs) not monitored by a data and safety monitoring board or committee are required to submit a Data Safety Monitoring (DSM) report to the CU Cancer Center DSMC at regular intervals based on risk as outlined in the institutional DSMP. This is a requirement from the FDA and the NCI to ensure that there is appropriate oversight for IITs under the purview of the CU Cancer Center. This does not include trials where the CU Cancer Center is not the coordinating center and the coordinating center is providing oversight.
These reports are submitted every 3, 6 or 12 months (as determined by DSMC) beginning approximately 6 months after the first participant is enrolled, and are due on the first of the month that the DSMC meets. The DSMC meets quarterly in January, April, July and October. This DSM reporting cycle may align with the annual IRB continuing review of the trial; therefore the DSM reporting cycle may be adjusted in order to allow for DSMC review and approval prior to continuing review submission. For trials where the CU Cancer Center investigator holds the IND or IDE, the report submitted to the FDA can be substituted for the DSM Progress Report.
Please contact the DSMC at [email protected] for access to the electronic DSM Progress Report and for any questions regarding the reporting process.
Committee Members
| Name | Title | Expertise |
| Peter Kabos, MD | Associate Professor (Chair) | Medical Oncology |
| Theresa Medina, MD | Assistant Professor | Medical Oncology |
| Steven Schuster, MD | Assistant Professor | Hematology/Medical Oncology (North Region) |
| Steven Bair, MD | Assistant Professor | BMT/Hematologic Malignancies |
| Heshman Eissa, MD | Physician | Oncology Clinic |
| Ryan Lanning, MD | Assistant Professor | Radiation Oncology |
| Elena Shagisultanova, MD | Assistant Professor | Medical Oncology |
| Marie Wood, MD | Professor | Hematology |
| Grace Bosma, MS | Research Instructor | Biostatistics |
| Catherine Sutherland | Research Services Manager | COMIRB |
| Adam Poust, PharmD | Pharmacy Manager | Investigational Pharmacy |
| Melinda McCaw, PharmD | Pharmacist | Investigational Pharmacy |
| Anna Blomberg, Pharm D | Pharmacist | Investigational Pharmacy |
| Terese Henry, PharmD | Pharmacist | Investigational Pharmacy |
Diana Abbott, PhD, MS | Research Associate | Biostatistics |
| Anna-Marie Locher, BS, CCRC | DSMC Manager | Data Safety Management |
| Jose Velez, PhD | DSMC Senior Auditor | Data Safety Management |