Shared Content Block:
Styles for CDB Faculty Pages
Rytis Prekeris, Ph.D.
Professor and Director, Molecular Biology Graduate Program

| [email protected] | |
| 303-724-3411 | |
| Ph.D., East Carolina University - Greenville, 1997 | |
| Prekeris Lab |
Graduate Program Affiliations:
Ongoing and Future Research
Molecular Mechanisms Regulating Cell Polarization and Protein Targeting
My general research interest is to understand the mechanisms of cell polarization and the functional consequences of this polarization during development and cancerogenesis. In the last several years my laboratory has focused on three distinct but related projects: regulation and function of asymmetric cell division, mechanisms of cancer metastasis and regulation of epithelia polarization in vitro and in vivo. The latest findings and future directions for these projects are described below.
.jpg?sfvrsn=9291bfba_2)
1. Molecular mechanisms of epithelial cells polarization
Epithelial cells are structurally and functionally polarized to transport specific molecules while maintaining a trans-epithelial barrier. This cellular asymmetry is essential for the proper functioning of epithelial tissues and depends on polarized endocytic transport routes. Additionally, epithelial cells coordinate their polarization with neighboring cells to form an apical lumen, a key step in the establishment of renal and gut architecture, and thereby function. Indeed, malfunctions in epithelial cell polarization and apical lumen formation are responsible for a variety of renal and intestinal disorders, such as polycystic kidney disease, renal tubular acidosis, microvilli inclusion disease and diabetes insipidus. This project aims to fill critical gaps in our understanding of epithelial cell biology, including what are the molecular mechanisms mediating coordinated formation of apical lumen during epithelia morphogenesis and function.
Despite recent advances in our understanding of the mechanisms mediating lumen formation, many questions remains unanswered. How are endosomes targeted during apical lumen formation? How do cells establish the site of single apical lumen? Do the mechanisms of lumen formation established in vitro also apply to epithelial morphogenesis in vivo? Addressing these pivotal and unanswered questions forms the backbone of this project. This study is designed to analyze the machinery of apical lumen formation using in vitro (MDCK cells three dimensional cultures) and in vivo (zebrafish) models.
2. Regulation breast cancer metastasis
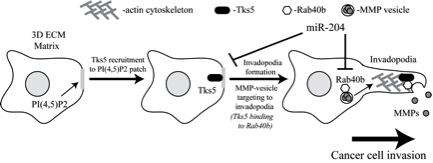
Remodelling of the extracellular matrix (ECM) is a key process during tumor growth and metastasis and is mediated via formation of structures, known as invadopodia, and targeted secretion of enzymes, known as matrix metalloproteinases (MMPs). Invadopodia are actin-rich structures that degrade the ECM and have been shown to be important for tumor progression. MMP2/9 also have been shown to be enriched at the invadopodia and are known for their roles in breast tumor growth/metastasis. As a result, MMP2/9 have emerged as possible therapeutic targets.
Unfortunately, clinical trials directly inhibiting MMP2/9 have proven ineffective, mostly due to adverse side effects and pre-existing high levels of secreted MMPs. It has been proposed that targeting the machinery specifically mediating MMP secretion at cancer cell invadopodia is more effective approach. However, little is known about targeted MMP2/9 secretion since most of the studies have focused on the mechanisms mediating ECM and cell adhesion and actin dynamics during metastasis. In contrast, very few studies have analysed the machinery mediating targeted MMP secretion in cancer cells in vitro and tested these mechanisms in vivo.
The main goal of this project is to combine studies using basic cell and molecular biology techniques with more translational in vivo approaches to define and systematically analyse novel pathways that mediate targeted MMP transport and secretion during breast cancer metastasis. The strength of this project is combining of the unique expertise from my lab (cell biology and Rab GTPases), Dr. Paul Jedlicka (microRNA and pathology) and Dr. Tracy Lyons (breast cancer and xenograft assays) laboratories. This combination will allow us to comprehensively test these hypotheses in vitro and in vivo.
3. The role of midbodies in regulating cancer and stem cell proliferation
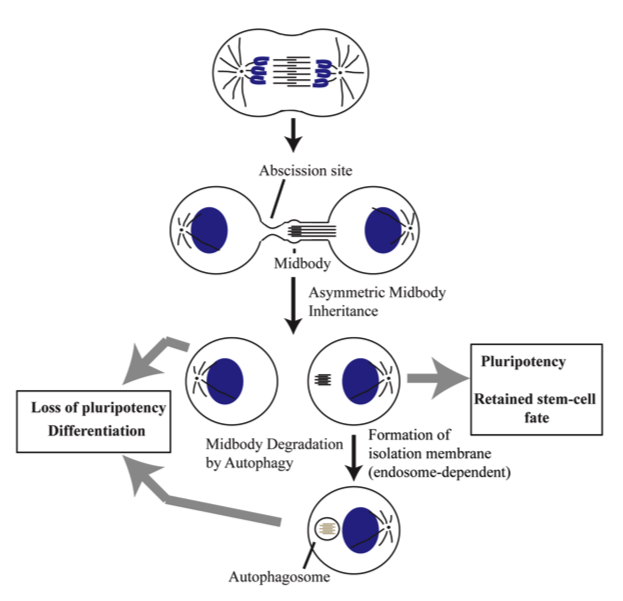
Cytokinesis is the final stage of the cell cycle resulting in physical separation of daughter cells. At late mitosis, the mother cell divides by the formation of a cleavage furrow, leaving two daughter cells connected by a thin intercellular bridge (ICB). The resolution of this bridge, abscission, is mediated by coordinated action between specialized recycling endosomes and cytoskeleton. During ingression of the cleavage furrow, the central spindle microtubules are compacted to form the structure known as the midbody (MB). The MB is situated within the ICB, with the abscission usually occurring at one side of the MB. As a result of this one-sided (asymmetric) abscission, only one daughter cell inherits the post-mitotic MB. These post-mitotic MBs can then either accumulate in the cytoplasm or be degraded. Interestingly, recent studies have identified post-mitotic MBs as novel signaling platforms regulating stem cell fate and proliferation. Indeed, stem cells were shown to accumulate post-mitotic MBs and the induction of MB accumulation leads to reprogramming of cell fate and conversion to highly-proliferative stem cell-like phenotypes. Despite the importance of MBs in determining cell fate and proliferative capacity, the mechanisms that regulate asymmetric MB inheritance and post-mitotic degradation remains completely unknown. As the result, the main goal of this project is to identify the factors mediating MB inheritance and accumulation, and to test the role of these factors in regulating stem cell fate and differentiation.
View Dr. Preteris's Latest Publications in PubMed
Duncan ED, Han KJ, Trout MA, Prekeris R. Ubiquitylation by Rab40b/Cul5 regulates Rap2 localization and activity during cell migration. J Cell Biol. 2022 Apr 4;221(4):e202107114. doi: 10.1083/jcb.202107114. Epub 2022 Mar 16. PMID: 35293963; PMCID: PMC8931537.
Farmer T, Prekeris R. New signaling kid on the block: the role of the postmitotic midbody in polarity, stemness, and proliferation. Mol Biol Cell. 2022 Mar 1;33(3). doi: 10.1091/mbc.E21-06-0288. PMID: 35179994.
Lencer E, Prekeris R, Artinger KB. Single-cell RNA analysis identifies pre- migratory neural crest cells expressing markers of differentiated derivatives. Elife. 2021 Aug 16;10:e66078. doi: 10.7554/eLife.66078. PMID: 34397384; PMCID: PMC8367380.
Linklater ES, Duncan ED, Han KJ, Kaupinis A, Valius M, Lyons TR, Prekeris R. Rab40-Cullin5 complex regulates EPLIN and actin cytoskeleton dynamics during cell migration. J Cell Biol. 2021 Jul 5;220(7):e202008060. doi: 10.1083/jcb.202008060. Epub 2021 May 17. PMID: 33999101; PMCID: PMC8129794.
Gibieža P, Peterman E, Hoffman HK, Van Engeleburg S, Skeberdis VA, Prekeris R. Rab14/MACF2 complex regulates endosomal targeting during cytokinesis. Mol Biol Cell. 2021 Apr 1;32(7):554-566. doi: 10.1091/mbc.E20-09-0607. Epub 2021 Feb 10. PMID: 33566684; PMCID: PMC8101466.
Jewett CE, Soh AWJ, Lin CH, Lu Q, Lencer E, Westlake CJ, Pearson CG, Prekeris R. RAB19 Directs Cortical Remodeling and Membrane Growth for Primary Ciliogenesis. Dev Cell. 2021 Feb 8;56(3):325-340.e8. doi: 10.1016/j.devcel.2020.12.003. PMID: 33561422; PMCID: PMC7880532.
Duncan ED, Lencer E, Linklater E, Prekeris R. Methods to Study the Unique SOCS Box Domain of the Rab40 Small GTPase Subfamily. Methods Mol Biol. 2021;2293:163-179. doi: 10.1007/978-1-0716-1346-7_11. PMID: 34453716; PMCID: PMC8455146.
Jewett CE, Appel BH, Prekeris R. The Rab11 effectors Fip5 and Fip1 regulate zebrafish intestinal development. Biol Open. 2020 Oct 23;9(10):bio055822. doi: 10.1242/bio.055822. PMID: 32973079; PMCID: PMC7595698.
Turn RE, East MP, Prekeris R, Kahn RA. The ARF GAP ELMOD2 acts with different GTPases to regulate centrosomal microtubule nucleation and cytokinesis. Mol Biol Cell. 2020 Aug 15;31(18):2070-2091. doi: 10.1091/mbc.E20-01-0012. Epub 2020 Jul 2. PMID: 32614697; PMCID: PMC7543072.
Peterman E, Valius M, Prekeris R. CLIC4 is a cytokinetic cleavage furrow protein that regulates cortical cytoskeleton stability during cell division. J Cell Sci. 2020 May 14;133(9):jcs241117. doi: 10.1242/jcs.241117. PMID: 32184265; PMCID: PMC7240295.
Peterman E, Prekeris R. The postmitotic midbody: Regulating polarity, stemness, and proliferation. J Cell Biol. 2019 Dec 2;218(12):3903-3911. doi: 10.1083/jcb.201906148. Epub 2019 Nov 5. PMID: 31690620; PMCID: PMC6891101.
Peterman E, Gibieža P, Schafer J, Skeberdis VA, Kaupinis A, Valius M, Heiligenstein X, Hurbain I, Raposo G, Prekeris R. The post-abscission midbody is an intracellular signaling organelle that regulates cell proliferation. Nat Commun. 2019 Jul 18;10(1):3181. doi: 10.1038/s41467-019-10871-0. PMID: 31320617; PMCID: PMC6639393.
Das L, Gard JMC, Prekeris R, Nagle RB, Morrissey C, Knudsen BS, Miranti CK, Cress AE. Novel Regulation of Integrin Trafficking by Rab11-FIP5 in Aggressive Prostate Cancer. Mol Cancer Res. 2018 Aug;16(8):1319-1331. doi: 10.1158/1541-7786.MCR-17-0589. Epub 2018 May 14. PMID: 29759989; PMCID: PMC6369592.
Antanavičiūtė I, Gibieža P, Prekeris R, Skeberdis VA. Midbody: From the Regulator of Cytokinesis to Postmitotic Signaling Organelle. Medicina (Kaunas). 2018 Jul 30;54(4):53. doi: 10.3390/medicina54040053. PMID: 30344284; PMCID: PMC6174351.
Jewett CE, Prekeris R. Insane in the apical membrane: Trafficking events mediating apicobasal epithelial polarity during tube morphogenesis. Traffic. 2018 May 16:10.1111/tra.12579. doi: 10.1111/tra.12579. Epub ahead of print. PMID: 29766620; PMCID: PMC6239989.
Gibieža P, Prekeris R. Rab GTPases and cell division. Small GTPases. 2018 Mar 4;9(1-2):107-115. doi: 10.1080/21541248.2017.1313182. Epub 2017 May 4. PMID: 28471300; PMCID: PMC5902206.
Dionne LK, Peterman E, Schiel J, Gibieža P, Skeberdis VA, Jimeno A, Wang XJ, Prekeris R. FYCO1 regulates accumulation of post-mitotic midbodies by mediating LC3-dependent midbody degradation. J Cell Sci. 2017 Dec 1;130(23):4051-4062. doi: 10.1242/jcs.208983. PMID: 29196475; PMCID: PMC5769594.
Mandell MA, Jain A, Kumar S, Castleman MJ, Anwar T, Eskelinen EL, Johansen T, Prekeris R, Deretic V. Correction: TRIM17 contributes to autophagy of midbodies while actively sparing other targets from degradation. J Cell Sci. 2017 Mar 15;130(6):1194. doi: 10.1242/jcs.202499. Erratum for: J Cell Sci. 2016 Oct 1;129(19):3562-3573. PMID: 28298613; PMCID: PMC6295128.
Peterman E, Prekeris R. Understanding post-mitotic roles of the midbody during cell differentiation and polarization. Methods Cell Biol. 2017;137:173-186. doi: 10.1016/bs.mcb.2016.04.001. Epub 2016 Apr 23. PMID: 28065304; PMCID: PMC5488711.
Jacob A, Linklater E, Bayless BA, Lyons T, Prekeris R. The role and regulation of Rab40b-Tks5 complex during invadopodia formation and cancer cell invasion. J Cell Sci. 2016 Dec 1;129(23):4341-4353. doi: 10.1242/jcs.193904. Epub 2016 Oct 27. PMID: 27789576; PMCID: PMC5201011.