Nathan E. Schoppa, PhD
Professor
Departmental Vice-Chair
/nathan-schoppa-(300-x-350).jpg?sfvrsn=b22aceba_2)
Department of Physiology and Biophysics
University of Colorado Anschutz
RC1 North Tower, P18-7100
Mail Stop 8307
Aurora, CO 80045
Tel (303) 724-4523
E-mail: [email protected]
Graduate Program Affiliations:
CV(Schoppa)2025
(pdf)
211 KB
Shared Content Block:
Physiology & Biophysics Styles -- Borderless table for directories
 Our
lab is interested in understanding mechanisms and function of brain circuits involved in processing olfactory information. Our focus is on two structures, the olfactory bulb and the piriform cortex, asking basic questions about what neurons are present,
how they are connected, and how groups of neurons work to effect a particular circuit output. Methodologically, we combine electrophysiological recordings in brain slices and in vivo in rodents, computational and ultrastructural approaches,
as well as behavioral studies. We also use transgenic/viral techniques for labeling specific cell-types and optogenetic manipulation.
Our
lab is interested in understanding mechanisms and function of brain circuits involved in processing olfactory information. Our focus is on two structures, the olfactory bulb and the piriform cortex, asking basic questions about what neurons are present,
how they are connected, and how groups of neurons work to effect a particular circuit output. Methodologically, we combine electrophysiological recordings in brain slices and in vivo in rodents, computational and ultrastructural approaches,
as well as behavioral studies. We also use transgenic/viral techniques for labeling specific cell-types and optogenetic manipulation.
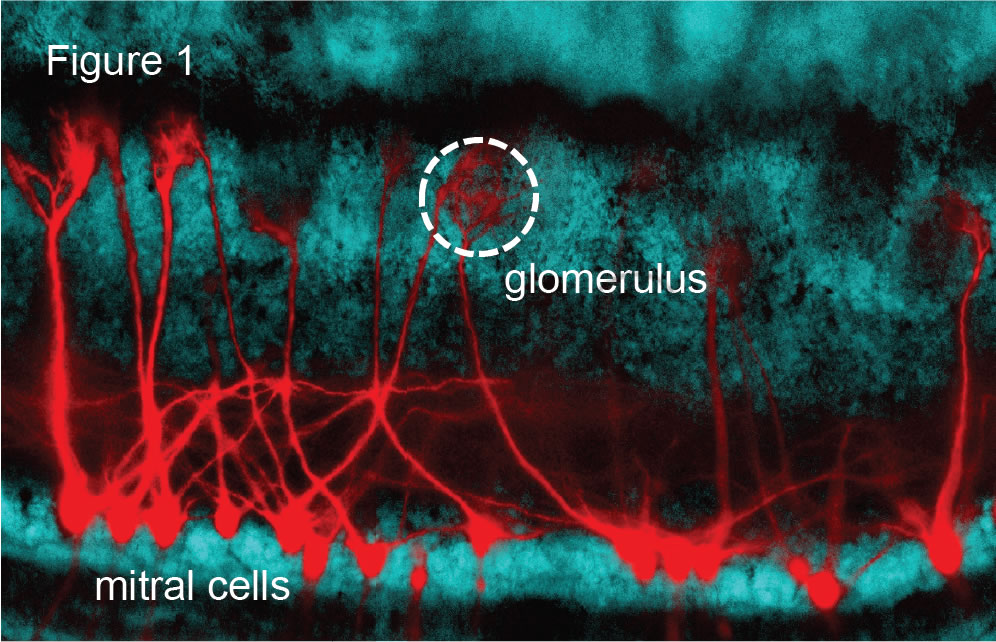
One specific current research interest in the lab is on the neuropil structures that line the outer surface of the olfactory bulb, called glomeruli (Figure 1). Glomeruli are the site of input into the bulb from axons of olfactory sensory neurons (OSNs). These structures are an especially attractive model for understanding general circuit function in the brain, as they are quite compact, about 80 µm in diameter, and also have a dedicated small population of cells with apical dendritic arbors restricted to one glomerulus. These include ~20 mitral cells, 50 tufted cells, and ~500 GABAergic periglomerular (PG) cells. A glomerulus also has a clear link to function, as each glomerulus encodes information about one type of odorant receptor in the nose. In studies of the organization of synapses in glomeruli, we have provided evidence that, in contrast to the prior prevailing view, there is a layer of processing involving both glutamatergic and GABAergic interneurons that impacts much of the signaling between OSNs and mitral cells and tufted cells. We are presently examining what impact this extra layer of processing has on the input-output relationship of a glomerulus. For example, do the identified mechanisms differentially impact mitral cells and tufted cells, causing the two cell-types to carry distinct information about an odor stimulus?
In addition to studying basic mechanisms in olfactory circuits, our lab is also examining olfactory dysfunction under disease conditions. In on-going studies, we are examining circuit defects in the olfactory bulb in a mouse model for Fragile X Syndrome ( Fmr1 KO mice) and the effect that these have on olfactory behavior. For studying the behavioral effects of Fmr1 KO, we use the classic go/no-go behavioral paradigm, modified in our experiments to assess the ability of a mouse to discriminate two odors.
Understanding the Brain’s Sense of Smell: Research That Could Fight Alzheimer’s
Current Lab Members
/praveen-kuruppath----100-x-125.jpg?sfvrsn=e1166ab9_4) | Praveen Kuruppath, PhD Research Associate |
Alumni
David Gire, PhD (Graduate student, 2005-2009)
Assistant Professor, Psychology, University of Washington
Tom McTavish, PhD (Graduate student, 2005-2009)
Senior Machine Learning Scientist, DHI Group
Victor Luna, PhD (Postdoc, 2006-2008)
Assistant Professor of Clinical Neurobiology (Psychiatry), Columbia University
Sruthi Pandipati Thomas, MD, PhD (Graduate student, 2007-2011)
Assistant Professor, Physical Medicine and Rehabilitation, Baylor College of Medicine
Jennifer Whitesell, PhD (Graduate student, 2008-2012)
Associate Director of Translational Models, Cajal Neurocience
David Sheridan, PhD (Postdoc, 2008-2012)
Associate Professor, Biology and Earth Science, Otterbein University
Joseph Zak, PhD (Graduate student, 2011-2015)
Assistant Professor, Biological Sciences, University of Illinois, Chicago
Frederic Pouille, PhD (Postdoc, 2012-2017)
Senior Research Associate, Barry Connor’s lab, Brown University
Shelly Jones, PhD (Graduate student, 2015-2020)
Budget and Policy Analyst, Colorado Office of State Planning and Budgeting
Bourne, J.N., and Schoppa, N. (2025) Ultrastructural contributions to extrasynaptic glutamatergic signaling in olfactory bulb glomeruli. J. Comp. Neurol. 533, e70034
Kuruppath, P., Xue, L., Pouille, F., Jones, S.T., and Schoppa, N.E. (2023) Hyperexcitability in the olfactory bulb and impaired fine odor discrimination in the Fmr1 KO mouse model of fragile X syndrome. J Neuroscience 43, 8243-8258.
Zak, J.D. and Schoppa, N.E. (2022) Neurotransmitter regulation rather than cell-intrinsic properties shape the high-pass filtering properties of olfactory bulb glomeruli. J Physiol 600, 393-417.
Zak, J.D. and Schoppa, N.E. (2021) Optical manipulations reveal strong reciprocal inhibition but limited recurrent excitation within olfactory bulb glomeruli. eNeuro 8(6), ENEURO.0311-21.2021
Jones S., Zylberberg, J., and Schoppa, N. E. (2020) Cellular and synaptic mechanisms that differentiate mitral and tufted cells into parallel output channels in the olfactory bulb. Frontiers in Cellular Neuroscience 14, 6144377.
Gire, D.H.*, Zak, J.D.*, Bourne, J.N., Goodson, N.B., and Schoppa, N.E. (2019) Balancing extrasynaptic excitation and synaptic inhibition within olfactory bulb glomeruli. eNeuro 6, 4. *Co-first authors.
Pouille, F. and Schoppa, N. E. (2018) Cannabinoid receptors modulate an olfactory bulb local circuit by cortical feedback. Frontiers in Cellular Neuroscience 12:47.
Pouille, F.*, McTavish, T.S.*, Hunter, L.E, Restrepo, D., and Schoppa, N.E. (2017) Intraglomerular gap junctions enhance interglomerular synchrony in a sparsely-connected olfactory bulb network. J Physiology 595, 5965-5986. *Co-first authors.
Bourne, J.N., and Schoppa, N. (2017) Three-dimentional synaptic analyses of mitral cell and external tufted cell dendrites in rat olfactory bulb glomeruli. J. Comp. Neurol. 525, 592-609.
Zak, J. D., Whitesell, J. D., and Schoppa, N. E. (2015) Metabotropic glutamate receptors promote disinhibition of olfactory bulb glomeruli that scales with input strength. J. Neurophysiol. 113, 1907-1920.
Sheridan, D. C., Hughes, A. R., Erdelyi, F., Szabo, G., Hentges, S. T., and Schoppa, N. E. (2014) Matching of feedback inhibition with excitation ensures fidelity of information flow in the anterior piriform cortex. Neuroscience 275, 519-530.
Whitesell, J. D., Sorensen, K. A., Jarvie, B. C., Hentges, S. T., and Schoppa, N. E. (2013) Inter-glomerular lateral inhibition targeted on external tufted cells in the olfactory bulb. J. Neurosci. 33: 1552-1563.
Pandipati, S. and Schoppa, N. E. (2012) Age-dependent adrenergic actions in the main olfactory bulb that could underlie an olfactory sensitive period. J. Neurophysiol. 108: 1999-2007.
Gire, D. H., Franks, K. M., Zak, J. D., Tanaka, K. F., Whitesell, J. D., Mulligan, A. A., Hen, R., Schoppa, N. E. (2012) Mitral Cells in the Olfactory Bulb Are Mainly Excited through a Multistep Signaling Path. J. Neurosci. 32: 2964-2975.
Pandipati, S., Gire, D. H., and Schoppa, N. E. (2010) Adrenergic receptor-mediated disinhibition of mitral cells triggers long-term enhancement of synchronized oscillations in the olfactory bulb. J. Neurophysiol. 104: 665-674.
Gire, D. H. and Schoppa, N. E. (2009) Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J. Neurosci. 29: 13454-13464.
Luna, V.M. and Schoppa, N.E. (2008) GABAergic interneurons control input-spike coupling in the piriform cortex. J. Neurosci. 28, 8851-8859.
Schoppa, N. E. (2006) AMPA/kainate receptors drive rapid output and precise synchrony in olfactory bulb granule cells. J. Neurosci. 26: 12996-13006.
Schoppa, N. E. (2006) Synchronization of olfactory bulb mitral cells by precisely-timed inhibitory inputs. Neuron 49: 271-283.