Sleep Research Program
 Dr. Brice McConnell, MD, PhD is currently director of the sleep research program at the CUACC. His lab is studying the mechanisms by which sleep impacts memory functions. This link between memory and sleep has been known for over 100 years, but scientists are just now beginning to understand how this process occurs within individual brain cells and among the connections between brain regions.
Dr. Brice McConnell, MD, PhD is currently director of the sleep research program at the CUACC. His lab is studying the mechanisms by which sleep impacts memory functions. This link between memory and sleep has been known for over 100 years, but scientists are just now beginning to understand how this process occurs within individual brain cells and among the connections between brain regions.
Dr. McConnell’s lab is particularly interested in understanding how sleep may protect the brain against Alzheimer’s disease. Poor sleep that occurs during the aging process can negatively impact memory functions and ultimately contribute to the development of Alzheimer’s disease. Identifying these sleep changes very early may provide opportunities to reduce risk and begin treatments well before the development of Alzheimer’s disease.
In support of this endeavor, Dr. McConnell’s team has recently discovered unique brain communication signals that occur while brain cells are processing memories1. His team has identified that changes in the brain’s memory processing that occur across the human lifespan, and he is now working to identify which changes in brain communication may predict the development of Alzheimer’s disease many years before memory symptoms occur. Ultimately, this information about the brain cell electrical activity that underlies memory function and brain communication may be used to measure treatment effects of medication. These sleep-dependent memory functions may also become a powerful target for new medications that aim to restore memory and prevent or treat Alzheimer’s disease. Keep reading to learn more about the relationship between sleep and memory.
How Does Sleep Impact Memory?
During sleep, our brains process memory information and “sculp” the physical representations of memory information at the cellular level. Specialized cellular connections between neurons, referred to as the synapses, are purposefully weakened during sleep to help “chisel away” unwanted cellular structures in the brain. Other synapses are retained and/or strengthened during sleep to build stronger connections within the brain’s “memory sculptures.”
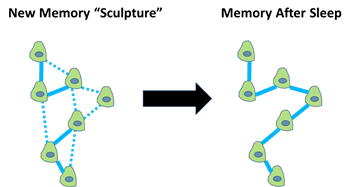
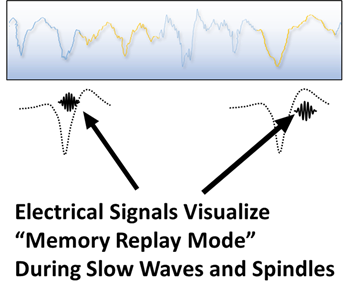
The processing of memory information during sleep occurs during a form of “playback mode” that is formally referred to as cortical reactivation. Modern neuroscience has allowed scientists to witness memories being replayed during sleep with the help of electrical recordings directly from the brain. The memory organizing center, termed the hippocampus, orchestrates each reactivation of memories during short bursts of electrical activity. These special electrical signals organize brain communication and transmit information between brain neurons.
Artificially disrupting sleep in an experimental setting interferes with memory performance, and preliminary studies suggest that it may be possible to boost the memory functions of sleep2-4. Dr. McConnell’s lab is particularly interested in how aging impacts this sleep and memory system, and his team recently discovered different types of electrical communication systems in the brain that seem to be vulnerable to the effects of aging1.
Watching the Brain Process Memories
 Dr. McConnell’s lab has been pioneering novel methods to observe the processing of memories during sleep. Electrical signals used by brain cells used for communication can be measured with the use of electroencephalography (EEG) as “brainwaves” emerging from inside of the brain to outside of the head. A simple EEG headband worn during sleep can provide recordings of brain waves that contain information about memory processing and brain communication.
Dr. McConnell’s lab has been pioneering novel methods to observe the processing of memories during sleep. Electrical signals used by brain cells used for communication can be measured with the use of electroencephalography (EEG) as “brainwaves” emerging from inside of the brain to outside of the head. A simple EEG headband worn during sleep can provide recordings of brain waves that contain information about memory processing and brain communication.
Knowing that the cortical reactivations of memory replay mode produce specific patterns of electrical activity, Dr. McConnell’s team developed automated methods to search for these patterns within the brainwaves from sleep EEG recordings. The “building blocks” of these patterns are electrical elements called slow waves and sleep spindles. Recent scientific studies have identified unique types of slow waves and spindles that are generated in different parts of the brain, and likely represent distinct types of memory processing during the cortical reactivations of sleep5,6.
The Connection Between Sleep and Alzheimer's Disease
The disruption of slow waves and spindles with the development of Alzheimer’s pathology (amyloid and tau accumulation) is a bi-directional relationship. Sleep disruption accelerates the development of Alzheimer’s pathology, and the presence of amyloid and tau disrupts the ability of the brain to create slow waves and spindles. This concept supports a neuroprotective role for sleep in preventing the development of Alzheimer’s disease, and creates a very novel treatment target for developing medications to fight Alzheimer’s (i.e., enhancing or restoring the neuroprotective aspects of sleep)7.
Changes in sleep may also occur very early in the process of developing Alzheimer’s disease, perhaps many years prior to the development of any memory problems. This suggests that sleep monitoring could provide a “read out” of brain health that predicts the future development of dementia. Dr. McConnell’s team is working to develop a predictive biomarker from a simple EEG headband that could be used by anyone in their home to monitor brain health. Ultimately, that tool might allow scientists to identify people who should be started on experimental medications to prevent or treat Alzheimer’s disease, and such a tool could also help monitor for how well any treatments are working on a day-to-day basis.
References
1. McConnell BV, Kronberg E, Teale PD, et al. The aging slow wave: a shifting amalgam of distinct slow wave and spindle coupling subtypes define slow wave sleep across the human lifespan. Sleep.
2. Diekelmann S, Born J. The memory function of sleep. Nature Reviews Neuroscience 2010;11(2):114.
3. Jha SK. Sleep, Memory and Synaptic Plasticity: Springer, 2019.
4. Rasch B, Born J. About sleep's role in memory. Physiological reviews 2013;93(2):681-766.
5. Jiang X, Gonzalez-Martinez J, Halgren E. Posterior Hippocampal Spindle Ripples Co-occur with Neocortical Theta Bursts and Downstates-Upstates, and Phase-Lock with Parietal Spindles during NREM Sleep in Humans. Journal of Neuroscience 2019;39(45):8949-8968.
6. Bernardi G, Siclari F, Handjaras G, Riedner BA, Tononi G. Local and widespread slow waves in stable NREM sleep: evidence for distinct regulation mechanisms. Frontiers in human neuroscience 2018;12:248.
7. Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer's disease? Trends in neurosciences 2016;39(8):552-566.

