Participate in a Study
Are you someone with Down syndrome who is interested in participating in research? Learn more about studies that are currently recruiting participants here.
Join our community!
Get our latest discoveries, events and research opportunities sent straight to your inbox.
Research on chronic constipation in individuals with Down Syndrome
The purpose of this study is to learn more about chronic constipation in Down syndrome to benefit those suffering from this condition and improve future treatments.
Anyone who has or does not have Down syndrome and has been diagnosed with chronic constipation that is not due to a known congenital condition may be eligible.
The Neurogastroenterology and Motility Program at Children's Hospital Colorado offers specialized treatments for chronic constipation including an expert motility physician, occupational and pelvic physical therapy, and a pediatric psychologist that are all typically covered by insurance.
Compensation is provided.
Collaborating Physician: Jaime Belkind-Gerson, MD, MSc
PI: Joaquin Espinosa, PhD
Want more information? Check out an expert Q&A featuring the study's collaborating physician Dr. Jaime Belkind-Gerson and investigator Dr. Kelly Sullivan.

Research on potential therapies to treat Down Syndrome Regression Disorder (DSRD)
The purpose of this research is to determine the safety and effectiveness of different potential treatments for DSRD.
Individuals with Down syndrome between the age of 8 and 30 years may be eligible to participate if they have symptoms or a diagnosis of DSRD. Participants must be accompanied by a study partner and agree to a randomized treatment assignment.
Compensation is provided and travel funds are available if needed.
PIs: Joaquin Espinosa, PhD; Elise Sannar, MD; Jonathon Santoro, MD | NCT05662228
Want more information? Check out our Down Syndrome Regression Disorder Clinical Trial research page to learn more about the science behind this study.

Research to develop the Human Trisome Project
The purpose of this study is to provide qualified and approved researchers with access to biological samples and health information to answer specific research questions. This project will significantly increase the speed of Down syndrome research and the understanding of associated medical conditions such as leukemia and Alzheimer’s disease.
Who can participate? Anyone 6 months to 89 years old who: (1) has Down syndrome, or (2) has a family member with Down syndrome, or (3) does not have Down syndrome.
PI: Joaquin Espinosa. COMIRB# 15-2170. NCT02864108.
Want more information? Check out our www.trisome.org website to learn more about the science enabled by the Biobank.

The Trial-Ready Cohort-Down Syndrome (TRC-DS)
As many as 9 in 10 people with Down syndrome develop Alzheimer’s disease.
The Trial-Ready Cohort-Down Syndrome, or TRC-DS, matches people with Down syndrome to clinical trials targeting Down-syndrome related Alzheimer’s disease. It is a national clinical study, and the Linda Crnic Institute for Down Syndrome is a qualified participation site near Denver, Colorado.
TRC-DS needs 120 healthy people between the ages of 25 and 55 with Down syndrome.
Because Alzheimer’s disease affects people with Down syndrome at a much earlier age than the general population, researchers are looking for volunteers 25 to 55 years old to participate in research.
Want more information? Check out our Trial-Ready Cohort-Down Syndrome (TRC-DS) research page to learn more.

Research studying early learning and biology in babies with Down syndrome
The Developmental Disabilities Research Laboratory at Colorado State University research is investigating the early connection between learning and biological factors in infants with Down syndrome.
This opportunity is for children with Down syndrome who are younger than 13 months old. Participation consists of one visit to a study site each year for three years. Study visits will include child play activities and caregiver questionnaires.
Compensation is provided.
Study Lead: Deborah Fidler, PhD COMIRB #41670895.0.
Want more information about the research team? Check out the Developmental Disabilities Research Laboratory page to learn more.

Research studying health care communication between adults with Down syndrome and doctors
Are you an adult with Down syndrome establishing care with a new primary care provider? You may be eligible to participate in a research study to help improve communication between patients, supporters, and doctors.
This study will examine how doctors, patients with Down syndrome, and their supporters talk to each other during clinic visits. The goal is to create a communication tool that makes these visits easier and more effective.
PI: Dr. Jessica Solomon Sanders. COMIRB #25-1679

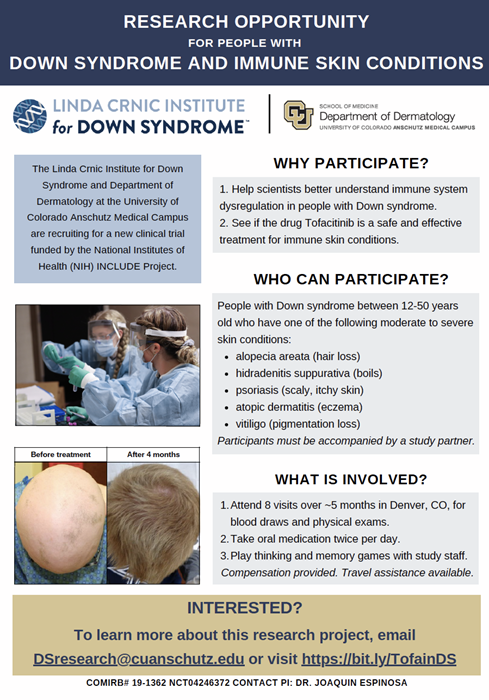
Research on tofacitinib to treat immune skin conditions in people with Down syndrome
The purpose of this research is to determine whether tofacitinib is a safe and effective treatment for immune skin conditions in people with Down syndrome, and to further our understanding of the immune system in Down syndrome.
Adolescents and adults with Down syndrome between the age of 12 and 50 years may be eligible to participate if they have an active immune skin condition. For example: eczema or atopic dermatitis, alopecia areata, hidradenitis suppurativa, psoriasis, or vitiligo.
Compensation is provided and travel funds are available if needed.
PI: Joaquin Espinosa. COMIRB #19-1362. NCT04246372.
Enrollment is complete.
Want more information? Check out our Immune Skin Conditions Clinical Trial research page to learn more about the science behind this study.