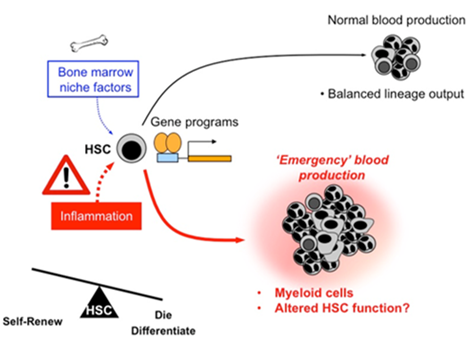
The goal of our lab is to use cutting-edge molecular, genetic and cellular approaches to understand how inflammation reprograms hematopoietic stem cell (HSC) fate choices, and to understand the short-and long-term functional consequences for HSCs exposed to inflammatory cytokines and other factors.
Our mission is to make discoveries that may lead to better therapeutic approaches for the treatment of deregulated blood production in disease conditions.
In our lab, we ask the following questions:
How does inflammation impact HSC fate to alter blood production?
How is HSC function impacted by chronic inflammatory disease?
Does chronic inflammation drive leukemia development and progression?
We know that inflammation has profound effects on the overall shape of the blood system, typically leading to overproduction of myeloid cells at the expense of other lineages. We are studying how cytokines such as IL-1 change the global transcriptional, epigenetic, and metabolic status of HSCs and the progenitor cells they differentiate into, thereby altering HSC fate and blood output.
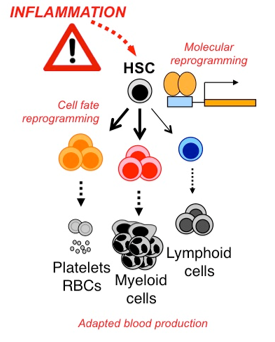
We are also addressing more broadly the role of other factors that signal inflammation, and how they might impact HSC function under normal and ‘emergency’ conditions. Collectively these studies will help us to better understand the biology of inflammation as a fundamental regulator of blood production, and identify key molecular and cellular pathways that control HSC responses to injury, infection and other insults.
Inflammation evolved as an ‘emergency’ response that is turned on and off quickly to avoid causing damage to our tissues. Nonetheless, it is a double-edged sword. Chronic inflammation, in which this response fails to turn off, is a key pathological component of a bewildering array of severe metabolic and autoimmune diseases, including atherosclerosis, obesity, diabetes and arthritis. Each of these conditions has distinct origins and pathologies, but all of them are marked by severely deregulated blood regulation that leads to:
- The overproduction of myeloid cells which propagates tissue damage
- Loss of erythrocyte production leading to anemia of chronic disease
- Greatly impaired immune system function that worsens the underlying disease and leaves patients vulnerable to infection.
We suspect the same inflammatory signals that activate HSCs in response to acute need alter their fate and function during chronic exposure, thereby creating a deregulated hematopoietic hierarchy that in essence underwrites the disease phenotype. We are therefore investigating the long-term consequences of chronic inflammation on HSC function using mouse models of these diseases, and whether blockade of inflammatory signals can normalize HSC function and reduce disease severity.
Many leukemias have been classically viewed to be the result of certain mutations that transform normal cells into leukemic stem cell (LSCs) clones, which in turn initiate and propagate disease. More recently, leukemia development is being viewed as a partnership between transforming mutations and deregulation of the microenvironment around the leukemic cells. Interestingly, many cancers, and in particular preleukemic clonal blood disorders such as myelodysplastic syndrome are associated with prior underlying chronic inflammatory disease.
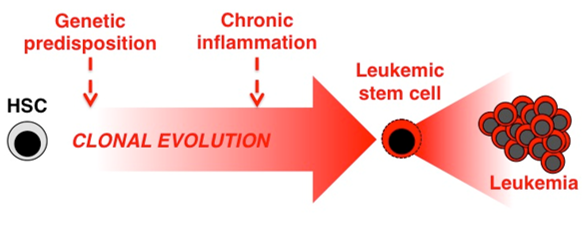
We suspect that chronic exposure to pro-inflammatory factors not only deregulates blood production by HSCs, but may also make them vulnerable to leukemic transformation. We also hypothesize that in the context of leukemia, inflammatory signals serve as growth and survival factors for LSCs. We are now investigating whether inflammation can drive cellular transformation and disease progression using several mouse genetics approaches, and whether these represent targets for novel anti-leukemia therapy.
More About Our Research
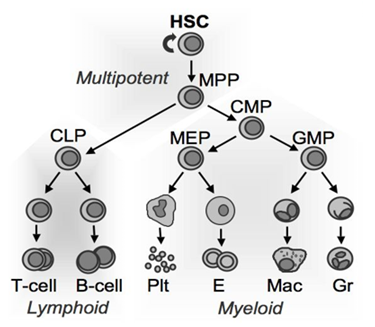
The blood system | A dynamic tissue
The blood is a fascinating liquid tissue, consisting of millions of cells distributed among several distinct lineages including myeloid and lymphoid leukocytes (white blood cells) that allow us to fight infections, erythrocytes (red blood cells) that carry oxygen from our lungs to the rest of our tissues, and platelets that clot wounds to prevent blood loss. Like all tissues, the millions of cells that make up our blood system (traditionally referred to as the hematopoietic system) are derived from a complex cellular hierarchy fed by a small population of self-renewing hematopoietic stem cells (HSCs for short) that actively build the blood system during fetal development and subsequently become dormant as adulthood sets in, activating periodically to maintain the blood system for the rest of our lives. Inherent in the ability of our blood systems to respond to these diverse requirements is the capacity to rapidly adapt to specific needs, such as producing more myeloid cells following infection, or more erythrocytes to respond to the slightly thinner atmosphere while visiting, or living in, Denver.
Inflammation | An ‘emergency’ alarm for HSCs
My laboratory’s research focuses on understanding how the blood system can adapt to these diverse needs. Since life isn’t perfect and most of us get sick, become injured, or otherwise make sudden and unexpected demands of our blood systems, dormant HSCs can be called to action. What has remained a mystery for some time is what physiological signals activate HSCs in this context, inform them what type of cells to overproduce, and shut them back down when the insult has passed.
During my post-doctoral training, I found that HSC activation is linked to the production of pro-inflammatory signals such as interferons (IFNs) and interleukin-1 (IL-1) in response to injury and infection. I’ve also studied how these signals alter the fate pathways HSCs choose when they become active, promoting cell death or differentiation into distinct hematopoietic progenitor cell populations, which eventually become new mature blood cells. From this work, we’ve learned that HSCs are not simply dormant cells insulated in their niches from the rest of the world, but instead are incredibly responsive to physiological changes, in particular, the ‘emergency’ signals generated during inflammation.