

Mission Statement
The immediate goal of our research is to improve the treatment and outcomes of patients with multiple myeloma, especially for patients who have developed difficult-to-treat multi-drug resistant disease. Our approach is to utilize primary bone marrow biopsies in developing new therapies, personalizing the application of current drugs, and designing novel drug combinations to treat acquired resistance. In the process, we seek to advance each other’s scientific and professional development within a fun, collaborative environment.
The sections below illustrate the core philosophies of our research program.
Multiple Myeloma
Multiple myeloma is a debilitating blood cancer that afflicts more than 30,000 Americans each year. There is much to be done to improve patients’ lives and finding a cure is achievable. Although the treatment has greatly improved in the past decade, no current therapy is capable of fully eliminating minimal residual disease in most patients. Myeloma is derived from plasma cells, which are responsible for antibody-based immunity. When they become cancerous, myeloma cells secrete monoclonal antibody that causes severe symptoms and also serves as a way to monitor the level of disease. Myeloma cells are susceptible to drugs that exploit their unique attributes, protease inhibitors, and IMiDs.
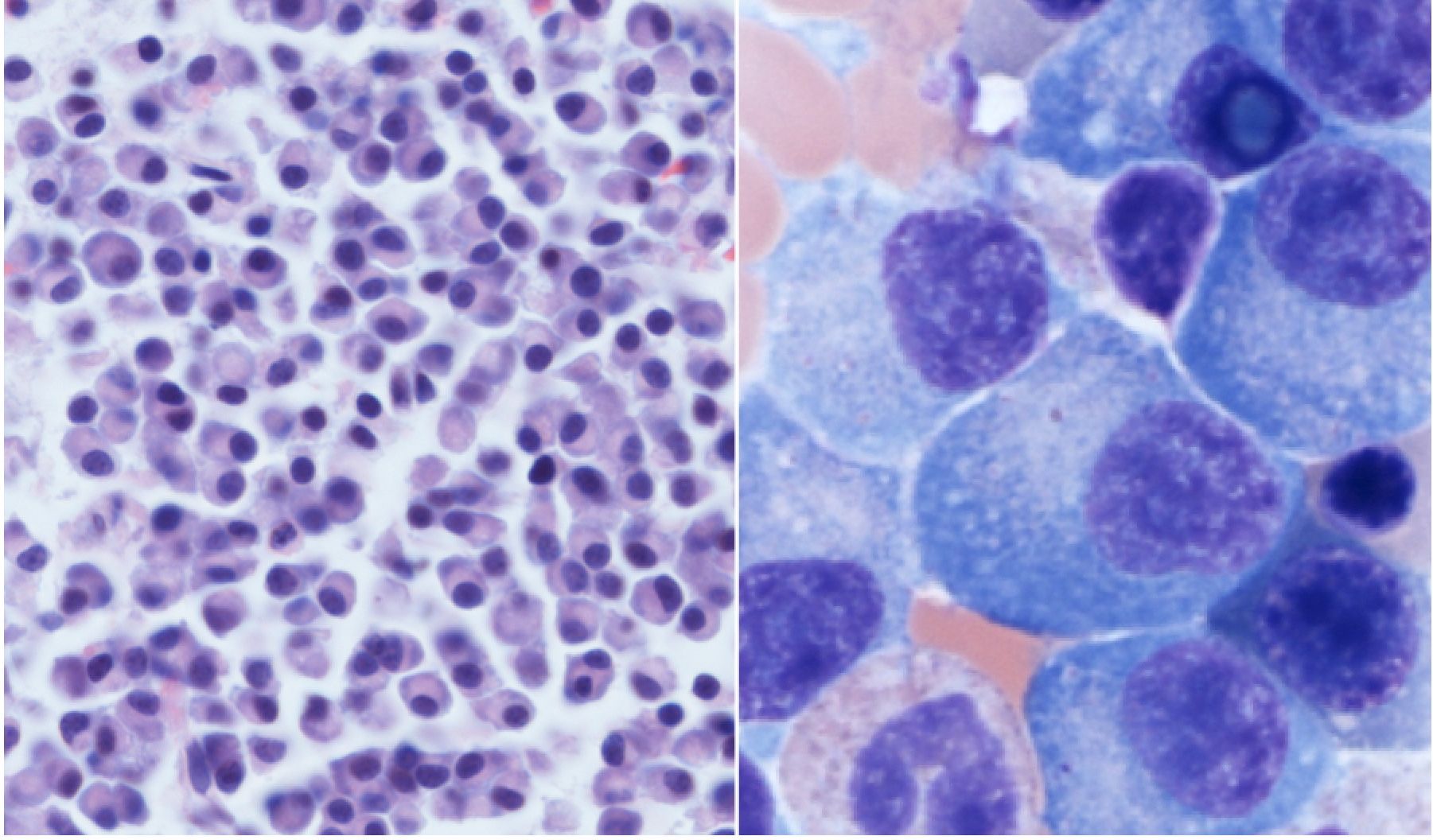
Myeloma cells under the microscope, crowding out the normal bone marrow cells. Photo credit to Jessica Davis, MD.
Antibody-Based Immunotherapies
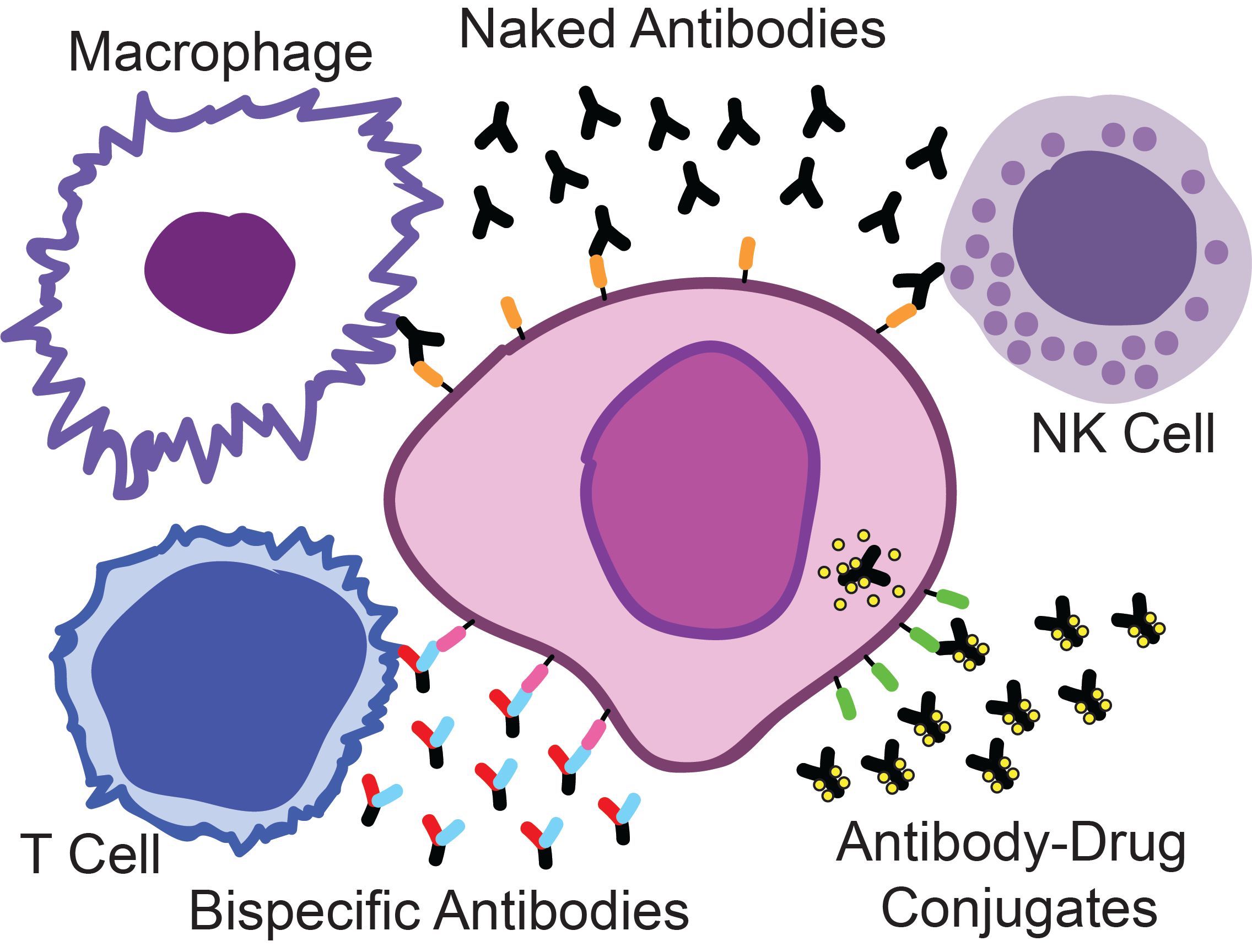
Naked antibodies attract components of the immune system, such as macrophages and natural killer (NK) cells to kill myeloma cells. Bispecific antibodies bind both myeloma and T-cells, activating the powerful killing activity of these cells. Antibody-drug conjugates are a targeted delivery system for highly potent chemotherapy directly into the myeloma cells.
Exciting breakthroughs have been made in the development of new immunotherapies for multiple myeloma. Myeloma is readily accessible to antibody-based therapies in the bone marrow and thus an ideal disease for this drug class. Recently, myeloma targeted naked antibodies have shown groundbreaking clinical activity. Now the potential for further improved clinical effects have emerged with antibody-drug conjugates and bispecific antibodies. Our focus is on the development of these antibody-based therapies and optimizing how they are applied, both in terms of finding patient subpopulations that will benefit most and in the rational design of drug combination strategies.
Patient-Donated Samples
The pipeline for new myeloma drugs is highly active, but completely dependent on cell line models, which don’t accurately reflect the disease in many ways. Thus, a priority for our lab is to bank tissue samples from patients with myeloma at UC Denver. Bone marrow aspirates are obtained clinically at diagnosis and relapse. Donation of an aliquot for research purposes allows us to examine a number of facets of disease biology and optimize its treatment. Among the most important technologies we apply is flow cytometry (aka FACS analysis). This technique allows the isolation of the malignant myeloma population from heterogeneous mixtures of cells for further genetic and phenotypic analyses.
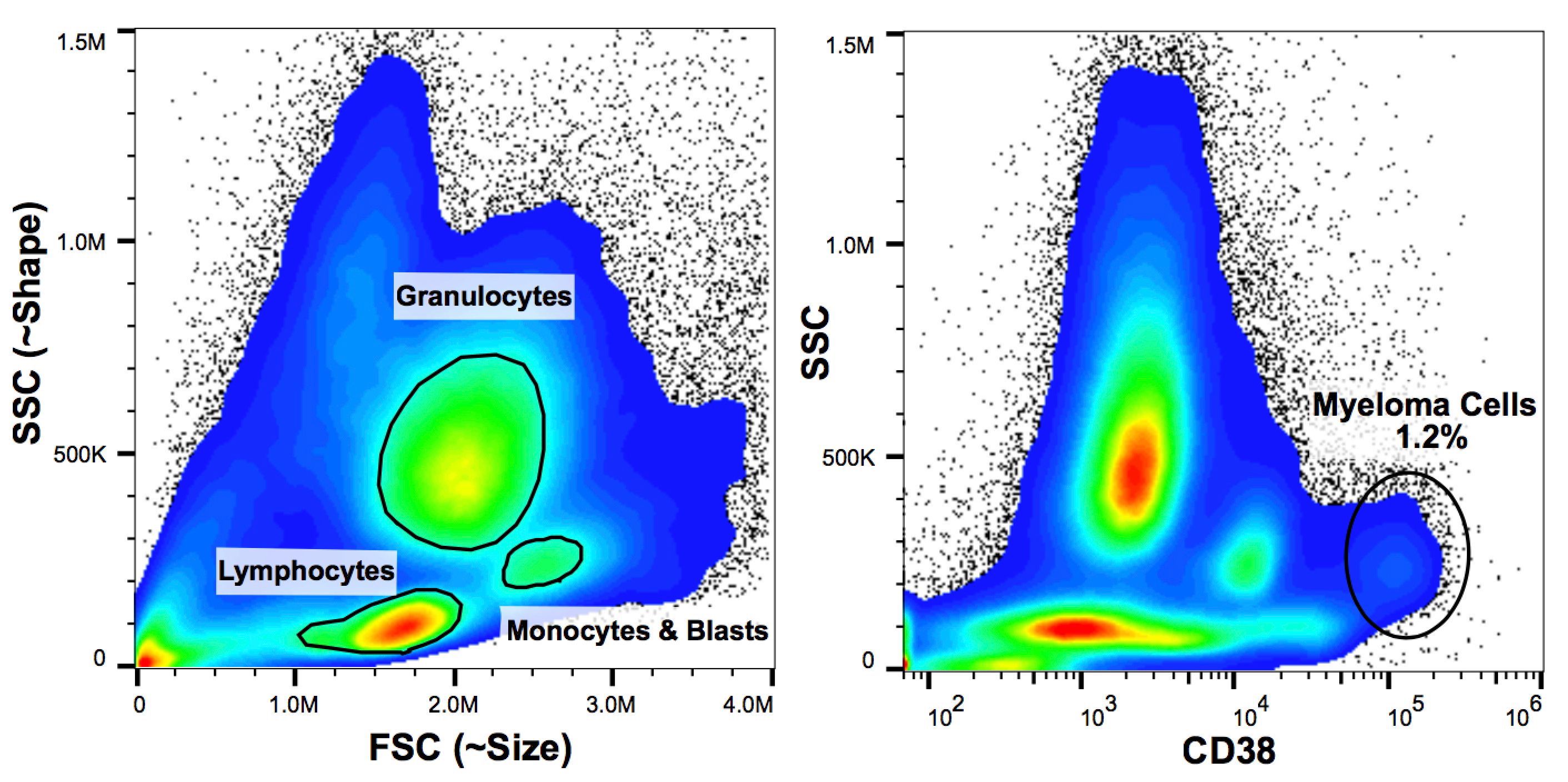
Flow cytometry of a bone marrow sample from a patient with myeloma. (left panel) Bone marrow contains a complex mixture of immune cells and blood cell progenitors that come in all shapes in sizes. (right panel) Differentiating myeloma cells from this mixture requires the use of multiple cell surface markers with known expression patterns on these cells, such as CD38.
Exploiting Protein Translation in Multiple Myeloma
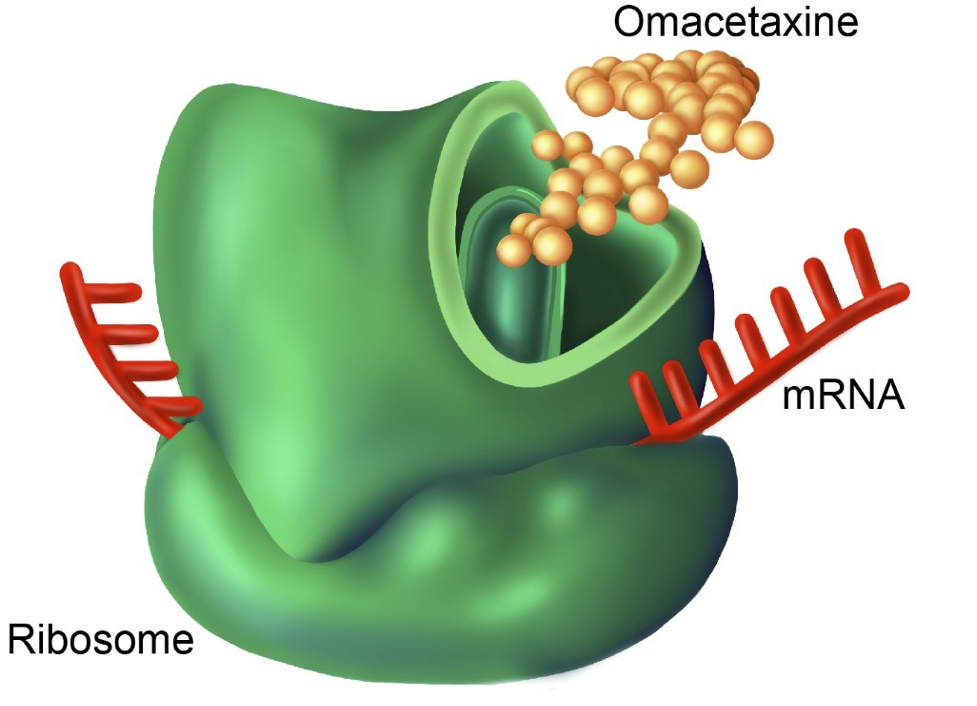
Illustration by Gwen Tice
Multiple myeloma cells, like the plasma cells they are derived from, display very high of the protein process required for antibody synthesis. Their dependence on this process causes rapid multiple myeloma cell death upon treatment with protein translation inhibitors, such as omacetaxine.
In pursuit of new therapies for myeloma patients, the lab has been investigating protein translation inhibition. Plasma cells produce large amounts of antibodies and as such have high baseline levels of protein translation compared to other cells in the bone marrow. A recent project from the lab has aimed to exploit these high translation levels by utilizing the protein translation inhibitor omacetaxine to kill myeloma cells. Omacetaxine is FDA approved for the treatment of chronic myeloid leukemia (CML). It has a unique mechanism of action among anti-cancer drugs: binding to the A-site of ribosomes blocking the initial elongation step of protein synthesis. We are currently investigating combination therapies with omacetaxine as well as exploring other translation inhibitors.
Targeting Myeloma
Currently, no therapy is capable of eliminating minimal residual disease (MRD), preventing cure, and inevitably leading to disease relapse in all patients. As the disease progresses, genetic subclones and subpopulations develop, some of which may underlie the emergence of multi-drug resistance. We believe understanding these developments is key to learning how to better control late-stage myeloma. Ultimately, we hope this type of study will facilitate the development of an approach capable of eliminating MRD, opening the possibility for the cure of this disease.
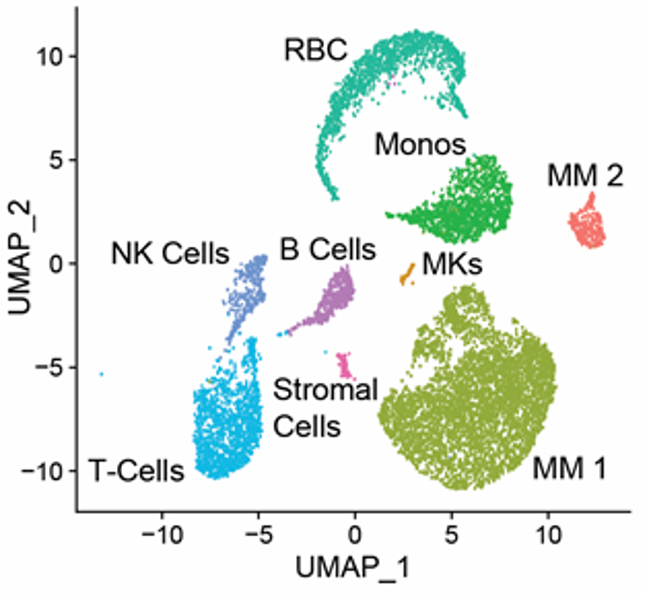
Single Cell RNA Sequencing of the bone marrow from a patient with multiple myeloma showing divergent subpopulations of malignant cells (labeled "MM1" and "MM2").



