Meet the Scientist
Dr. Julie Cooper
Professor & Chair
Department of Biochemistry & Molecular Genetics


How do our chromosomes – the structures that hold our DNA – make copies?
In each cell of your body, DNA is stored in structures called chromosomes. When cells divide these chromosomes are copied but over time, this copying process isn’t perfect. After many cycles of making copies, the ends of the chromosomes become shorter and can sometimes get damaged. Thankfully, we have telomeres, which are like protective caps on the ends of our chromosomes that help to decrease this damage when cells divide.
Think of your DNA as important paperwork that you need to keep copying for work and the telomeres as the paper folders that you keep the copies in to protect them from damage. However, over years of opening and closing and moving the folders around your office, the edges of the folders get beat up and ragged, eventually not protecting the copies of paperwork inside them very well over time. This is exactly what happens to telomeres!
Dr. Julie Cooper leads a research team at the University of Colorado Anschutz Medical Campus in the Department of Biochemistry and Molecular Genetics that studies the functions of the telomeres or protective caps on the ends of chromosomes. Their findings may help us to better understand aging and molecular processes that contribute to diseases like cancer.
Think of your DNA as important paperwork that you need to keep copying for work and the telomeres as the paper folders that you keep the copies in to protect them from damage. However, over years of opening and closing and moving the folders around your office, the edges of the folders get beat up and ragged, eventually not protecting the copies of paperwork inside them very well over time. This is exactly what happens to telomeres!
Dr. Julie Cooper leads a research team at the University of Colorado Anschutz Medical Campus in the Department of Biochemistry and Molecular Genetics that studies the functions of the telomeres or protective caps on the ends of chromosomes. Their findings may help us to better understand aging and molecular processes that contribute to diseases like cancer.
What is the ‘end replication problem’?
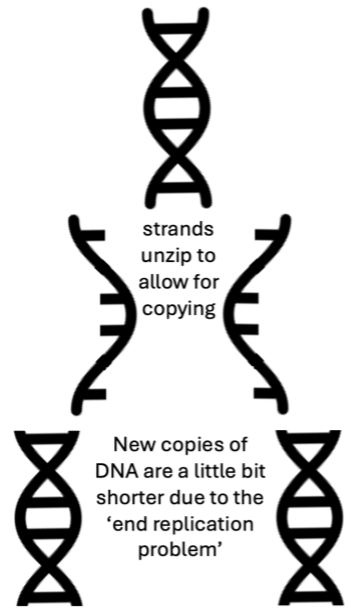
DNA is made up of two strands of molecules that are wound around each other. During cell division, these strands need to be ‘unzipped’ so each single strand can be used to create a new copy of DNA. However, the molecular machine that makes new DNA can’t start from scratch; the machine needs to latch onto the tip of an existing DNA strand at the end of chromosomes to get started. This process leaves the DNA that the machine bound to get the process started uncopied, creating a problem where the DNA at the ends of the chromosome is left uncopied. This is called the ‘end replication problem’ since the ends of the chromosomes don’t get copied where the machine bound to start the process.
Every time a cell divides, the telomeres get shorter but the important DNA farther inside the chromosome is protected and gets copied. However, once the telomeres become too short, the cell stops dividing because the genetic information further down the chromosome is no longer protected. Telomere shortening is part of what happens as we age, and scientists like Julie and her research team are studying how this process may play a role in the development of certain diseases like cancer.
Every time a cell divides, the telomeres get shorter but the important DNA farther inside the chromosome is protected and gets copied. However, once the telomeres become too short, the cell stops dividing because the genetic information further down the chromosome is no longer protected. Telomere shortening is part of what happens as we age, and scientists like Julie and her research team are studying how this process may play a role in the development of certain diseases like cancer.
So what exactly are telomeres?
Telomeres are repeated DNA sequences at the ends of chromosomes that provide many important functions. Telomeres attract special proteins (called telomere-binding proteins) that, along with the telomeres they bind to, protect our DNA in several ways. One of the most important functions of telomeres is to act as a protective cap to hide the ends of the chromosomes from being detected as damage-induced broken ends. Damage-induced broken ends in our chromosomes occur regularly from a variety of things like environmental pollutants or spontaneous chemical reactions in our cells. Normally, our cells will repair these breaks by fusing the ends together or, in other cases, chewing away a small amount of DNA at the break site to prepare it for the repair machinery to come in and fix the damage. But the natural ends of chromosomes have to avoid these types of repairs to ensure that important genetic information is not lost or scrambled.
So telomeres need to be protected not only from the end replication problem but also from enzymes within the repair machinery that chew away DNA from broken chromosome ends as well as enzymes that fuse broken ends together. Telomeres protect our genome - or complete set of genetic instructions - from all of these scenarios. However, when telomeres are defective or not working properly, chromosome fusion and degradation can result in the genome becoming unstable, which can speed up the aging of the cell and drive the development of cancer.
So telomeres need to be protected not only from the end replication problem but also from enzymes within the repair machinery that chew away DNA from broken chromosome ends as well as enzymes that fuse broken ends together. Telomeres protect our genome - or complete set of genetic instructions - from all of these scenarios. However, when telomeres are defective or not working properly, chromosome fusion and degradation can result in the genome becoming unstable, which can speed up the aging of the cell and drive the development of cancer.
What about telomerase?
The end replication problem means that telomeres become a little shorter each time our cells divide. However, some of our cells produce an enzyme called telomerase that replenishes the telomere sequences that are lost due to the end replication problem. Telomerase is an unusual enzyme – it does the complete opposite of what our cells do every day, which is use our DNA to make RNA to make proteins. Telomerase has a special function that no other enzyme has – it copies RNA into DNA! Because this is the complete opposite - or reverse - of what normally happens, it is called a reverse transcriptase. To carry out this unique function, it carries its own section of RNA that is used as a template to make the telomeric DNA sequence.
So what cells use telomerase? Telomerase is only ‘turned on’ and used in a small subset of cells in the human body, specifically our germline (i.e. egg and sperm) and stem cells. All of the other cells in the body use the breakdown of the telomeres as a ‘clock’ that limits the lifespan of the cell by only allowing them to replicate so many times before they die. When the telomeres in a cell become too short to protect a chromosome end, the cells stop dividing, a process that contributes to human aging. Interestingly, telomerase is reactivated and inappropriately turned ‘on’ in most cancer cells, which contributes to cancer cells becoming ‘immortal’ and able to replicate continuously.
So what cells use telomerase? Telomerase is only ‘turned on’ and used in a small subset of cells in the human body, specifically our germline (i.e. egg and sperm) and stem cells. All of the other cells in the body use the breakdown of the telomeres as a ‘clock’ that limits the lifespan of the cell by only allowing them to replicate so many times before they die. When the telomeres in a cell become too short to protect a chromosome end, the cells stop dividing, a process that contributes to human aging. Interestingly, telomerase is reactivated and inappropriately turned ‘on’ in most cancer cells, which contributes to cancer cells becoming ‘immortal’ and able to replicate continuously.
How do Julie and her research team answer their questions in the lab?
Julie’s team use a variety of different lab techniques to study telomeres like introducing mutations into different elements of the molecular machinery that play a role in copying to observe what happens, and microscopy to look at the different shapes and sizes of chromosomes by eye.
Through these approaches, the team has made some intriguing discoveries. For instance, they found that not only can defective telomeres be fused together, but also they can become entangled, leading to chaotic cell division. They have also found unexpected functions of telomere during meiosis, the process that forms egg and sperm cells, when telomeres cluster at the edges of the nucleus and choreograph chromosome movements.
Understanding how telomeres work will help scientists like Julie to better understand aging, cancer, and even infertility.
Through these approaches, the team has made some intriguing discoveries. For instance, they found that not only can defective telomeres be fused together, but also they can become entangled, leading to chaotic cell division. They have also found unexpected functions of telomere during meiosis, the process that forms egg and sperm cells, when telomeres cluster at the edges of the nucleus and choreograph chromosome movements.
Understanding how telomeres work will help scientists like Julie to better understand aging, cancer, and even infertility.
If you want to learn more about the scientist, please head to their official CU webpage.