Diabetic Eye Disease
According to recent data from the National Eye Institute,
more than 40% of Americans with diabetes already have some stage of diabetic retinopathy. Patients with diabetes are also at a 2 to 5-fold increased risk for cataract. Damage to these tissues results from biochemical and metabolic alterations occurring
in response to chronic hyperglycemia. Our studies have shown that products of aldose reductase, the first enzyme of the polyol pathway, may represent a biochemical link between hyperglycemia and diabetic complications in the eye and other major 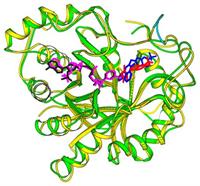 organ
systems. Inhibition of aldose reductase offers promise as a means to delay the onset or progression of diabetic complications.
organ
systems. Inhibition of aldose reductase offers promise as a means to delay the onset or progression of diabetic complications.
Recent studies have shown that aldose reductase is a member of a large enzyme superfamily of aldo-keto reductases. These enzymes may play a role in the disease process leading to blindness in diabetic patients. To better understand the potential involvement
of the AKRs in diabetic complications, we have begun using genetic techniques to dissect out the important players. Crystallography of recombinant AKRs showed the structural organization of AKRs as b/a barrel proteins and revealed functional side
chains involved in catalysis and the binding site for powerful enzyme inhibitors. We first used yeast genetics as a powerful genetic model to probe the functional conservation of AKRs. Our studies demonstrated that aldose reductase, the prototypical
AKR in humans, can rescue phenotypes in mutant yeast that had been genetically depleted of their AKR genes. We then developed mutant mouse models to further explore the role of this enzyme family in eye disease. Studies with transgenic mice showed
a definitive role for aldose reductase in diabetic cataracts. These animals were also useful to demonstrate a role for aldose reductase in retinal inflammation mediated by retinal microglia.
We have turned to natural products as potential sources of novel compounds showing potential as therapeutic agents against diabetic eye disease. Small molecules from Indian gooseberry and goji berry have shown particular promise against some of the major
markers associated with diabetic cataract and retinopathy.
Lens and Cataract Research
The lens is a remarkable tissue that is responsible for diffracting light so that it focuses precisely on the retina. Proper functioning of the lens requires it to be optically transparent, allowing light to travel unimpeded through the ocular media to
reach its target. To meet the demands of near and far vision, the lens also must be pliable so that the surface curvature can change when needed to adjust the focal distance. Vision problems arise when the lens loses its transparency (cataract) or
diminishes in its ability to change shape (presbyopia). Although cataract surgery is safe and effective, it is not without complications. As a consequence of the sheer number of surgical procedures performed annually in the United States in recent
years, cataracts were the most expensive eye disease, costing the Medicare budget more than any eye or vision condition with a medical diagnosis.
In a modern cataract procedure, the surgeon removes the cataractous lens by phacoemulsification. In this procedure, the hard lens nucleus is fragmented into small pieces using a narrow probe with a tip that vibrates at ultrasonic frequency. After aspiration
of the resulting fragments of lens nucleus, as well as tissue from the outer cortex, an artificial intraocular lens (IOL) is placed into the ‘‘empty’’ capsular bag and the patient’s vision restored after a recovery period
of a few hours. Although cataract surgery is considered a safe and effective surgical procedure, it can be conservatively estimated that up to 20% of cataract cases develop posterior capsular opacification (PCO), which results in a loss of clear vision
following a period of weeks to months after surgery. After the phacoemulsification procedure, a thin layer of lens epithelial cells (LECs) usually remain adherent to the inside of the capsular bag. A portion of these residual LECs become stimulated
to proliferate and migrate along the inner lining of the capsular bag until they reach the posterior aspect of the capsule. There the LECs continue to proliferate and become stacked into a disorganized mass of cells and induce wrinkling and contraction
of the capsule. In addition, LECs can migrate over the surfaces of the IOL and proliferate to form masses of cells. PCO results when the site of LEC accumulation and/or wrinkling of the capsular bag falls in the optical axis. The disorganized cell
masses and irregularities in the surface of the capsular bag cause scattering of light that would otherwise be focused on the retina, resulting in blurry vision and loss of visual acuity.
Fortunately, there is an effective outpatient procedure to deal with PCO. To remove the light-scattering elements in the optical axis in the PCO eye, ophthalmologists can give patients a noninvasive clinical procedure involving a brief exposure of the
eye to light emitted from a neodymium-doped yttrium aluminum garnet (Nd:YAG) laser. The laser emits short pulses of light energy that create a hole in the posterior capsule (YAG capsulotomy). With cells and their substrate now removed from the optical
axis, light can be appropriately diffracted to the retina to restore clear vision. Although the YAG procedure is noninvasive and fast, it is not without complications, which can include retinal detachments, retinal edema, and induction of spikes in
IOP. Therefore, YAG capsulotomy may not be suitable for patients who have a history of retinal disease or glaucoma.
Given the possible complications and costs associated with treating PCO, there is a critical need to understand the cellular mechanism that leads to PCO and possible ways to target this complication with either pharmaceutical strategies or by the development
of new technologies in IOL materials and design. Our research focuses on growth factor-mediated wound response pathways that become activated following cataract surgery. We and our collaborators are working to develop therapeutic agents to block the
signaling pathways that lead to PCO development. Much remains to be accomplished to bring possible new therapies to clinical trials and eventually to the clinic.
We also discovered that aldose reductase mediates signaling by TGF-b, a key driver of epithelial-to-mesenchymal transition in the pathogenesis of posterior capsule opacification.
Lens Regeneration
One the exciting and unexpected new developments in our work is the discovery of novel therapeutic agents to stimulate regrowth of the lens following cataract surgery. While replacement of the natural lens with an artificial lens is the most common practice
in adult patients, young patients are generally not well-suited for IOLs because their eyes and visual system are still growing and changing. We are working on therapeutic agents which show promise in stimulating the ability of individuals to regenerate
their own lenses following cataract surgery. We are developing technology needed to deliver therapeutic molecules to the eye in order to trigger lens regeneration in the young patient.
 Diabetes is a debilitating disease affecting millions of people worldwide, and is one of the leading causes of blindness among Americans over the age of 40. Diabetic eye disease often goes undetected in the early stages, and can lead to vision loss if not treated. We seek to develop novel therapies to prevent the beginning stages of diabetic eye disease, particularly for patients who develop diabetes early in life. With our team of collaborators, we have discovered several natural compounds in plants that show promise in their ability to prevent some of the early steps of diabetic eye disease. As these types of materials are developed from natural constituents of fruits and berries, they may satisfy the need for therapies that are safe and effective for patients who are faced with a life-long need for protection. Our goal is to rapidly translate our discoveries into new therapies that can be delivered to patients worldwide. Our research also addresses therapies to protect against cataract, a condition that has no medical means of prevention but still represents the world’s leading cause of blindness. We seek to identify new ways to prevent biochemical changes in the eye that underlie cataract formation, and to develop new medicines that may postpone or completely eliminate the need for cataract surgery.
Diabetes is a debilitating disease affecting millions of people worldwide, and is one of the leading causes of blindness among Americans over the age of 40. Diabetic eye disease often goes undetected in the early stages, and can lead to vision loss if not treated. We seek to develop novel therapies to prevent the beginning stages of diabetic eye disease, particularly for patients who develop diabetes early in life. With our team of collaborators, we have discovered several natural compounds in plants that show promise in their ability to prevent some of the early steps of diabetic eye disease. As these types of materials are developed from natural constituents of fruits and berries, they may satisfy the need for therapies that are safe and effective for patients who are faced with a life-long need for protection. Our goal is to rapidly translate our discoveries into new therapies that can be delivered to patients worldwide. Our research also addresses therapies to protect against cataract, a condition that has no medical means of prevention but still represents the world’s leading cause of blindness. We seek to identify new ways to prevent biochemical changes in the eye that underlie cataract formation, and to develop new medicines that may postpone or completely eliminate the need for cataract surgery. organ
systems. Inhibition of aldose reductase offers promise as a means to delay the onset or progression of diabetic complications.
organ
systems. Inhibition of aldose reductase offers promise as a means to delay the onset or progression of diabetic complications.