Joseph A. Brzezinski IV, Ph.D.
Associate Professor of Ophthalmology
Director, CellSight Laboratory of Developmental Genetics
 Background
Background
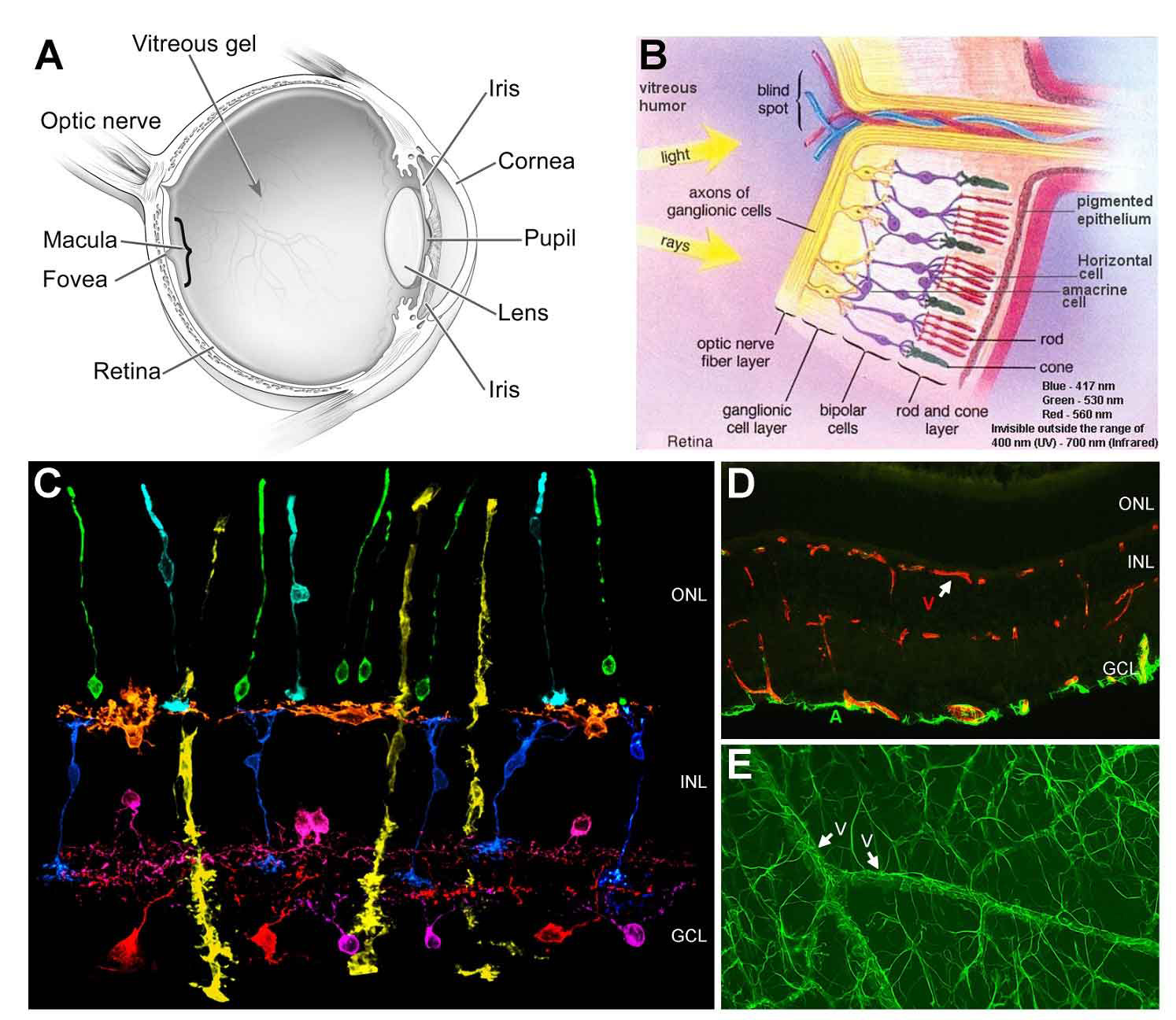
The retina is a thin nervous tissue that lines the back of the eye. It is responsible for detecting and relaying light stimuli to the brain. The retina is made up of seven principal cell types arranged into three layers (Figure 1). These include rod and cone photoreceptors; amacrine, bipolar, and horizontal interneurons; retinal ganglion cells (RGCs); and Müller glia. Rod photoreceptors detect dim light stimuli, mediating night vision. They are the most abundant cell type in the human retina and are located closest to the back of the eye, near the retinal pigmented epithelium (RPE). Cone photoreceptors are responsible for high acuity vision (like reading) and color discrimination. There are three types of cones in the human retina, each attuned to a specific wavelength of light (blue, green, red). Photoreceptors send their signals via bipolar interneurons, which are then relayed to the brain by the ganglion cells. Signaling between photoreceptors, bipolars, and ganglion cells is further modified by horizontal and amacrine cell interneurons. The retina-spanning Müller glia maintain retinal neuron health and establish a blood-retinal barrier between the vasculature and the neurons.
On a per weight basis, the retina is the most metabolically active tissue in the body. To satisfy this need for nourishment, the retina is perfused by blood vessels that form three planar layers (plexi). One plexus is on the inner surface of the retina and two capillary plexi are located deeper inside the retina (Figure 1). These vessels are made up of vascular endothelial cells and mural cells. Retinal astrocytes coat the surface of the retina and form a blood-retinal barrier with the surface vasculature (Figure 1)
Millions of Americans have vision loss caused by diseases that affect the retina (Figure-2). These diseases can affect neurons directly or other structures in the eye such as the vasculature or the RPE. If the disease process causes the death of retinal neurons, permanent vision loss can occur since neurons do not regenerate. For example, age-related macular degeneration (AMD) results in permanent loss of photoreceptors and significant visual impairment due to RPE and/or vascular alterations (Figure 3). Patients with glaucoma (Figure 2) permanently lose their ganglion cells and cannot relay photic information to the brain. Less commonly, there are diseases caused by defects in genes that control the development and/or function of specific populations of retinal neurons. For example, some genetic mutations cause retinitis pigmentosa, where photoreceptors are progressively lost (Figure 2). Restoring vision in these patients is our long-term goal (see CellSight). One promising way to do this is to replace lost retinal neurons with those grown from stem cell sources. The key to accomplishing this type of treatment is understanding the genetic instructions that tell retinal neurons how to form during development.
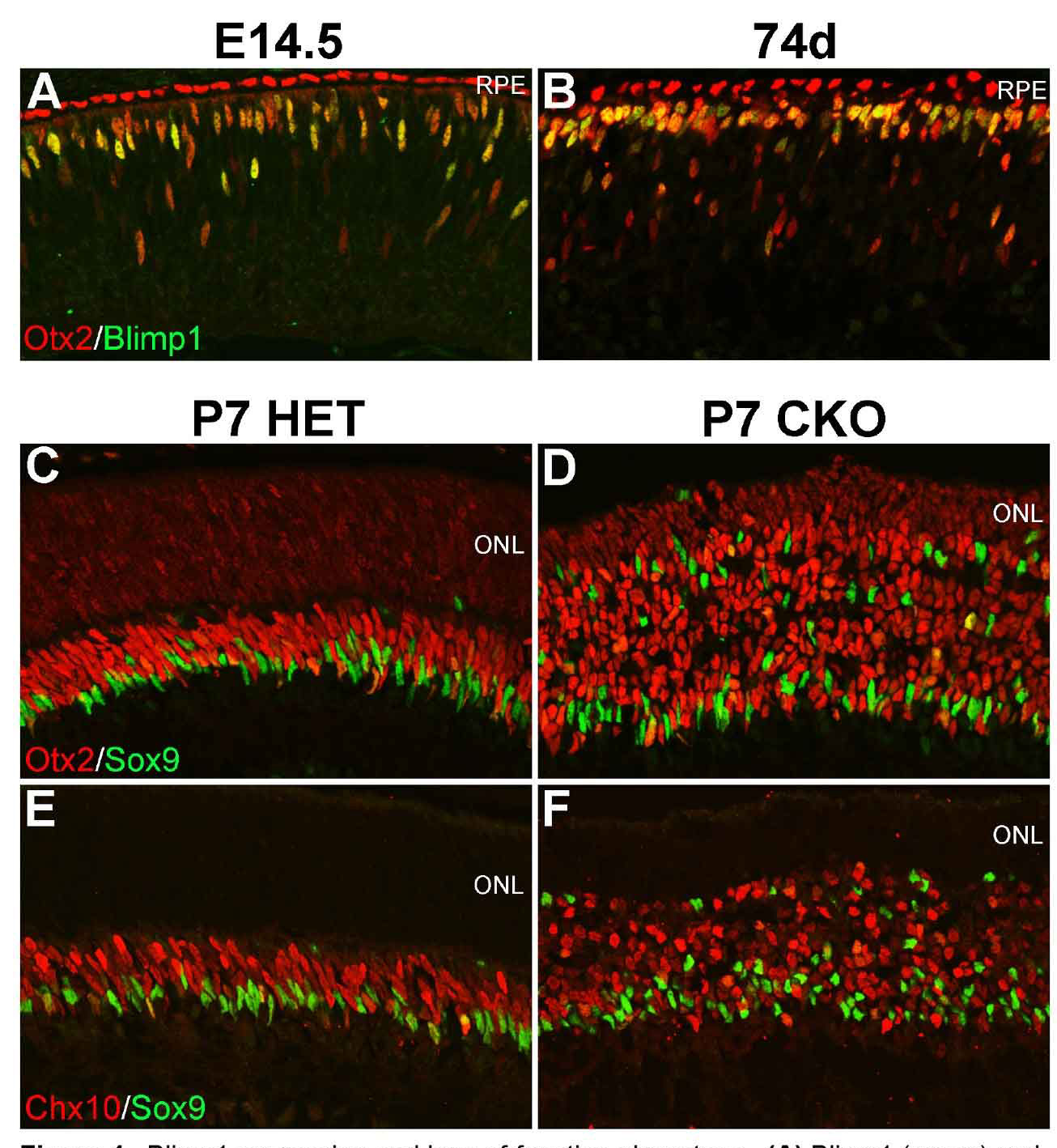
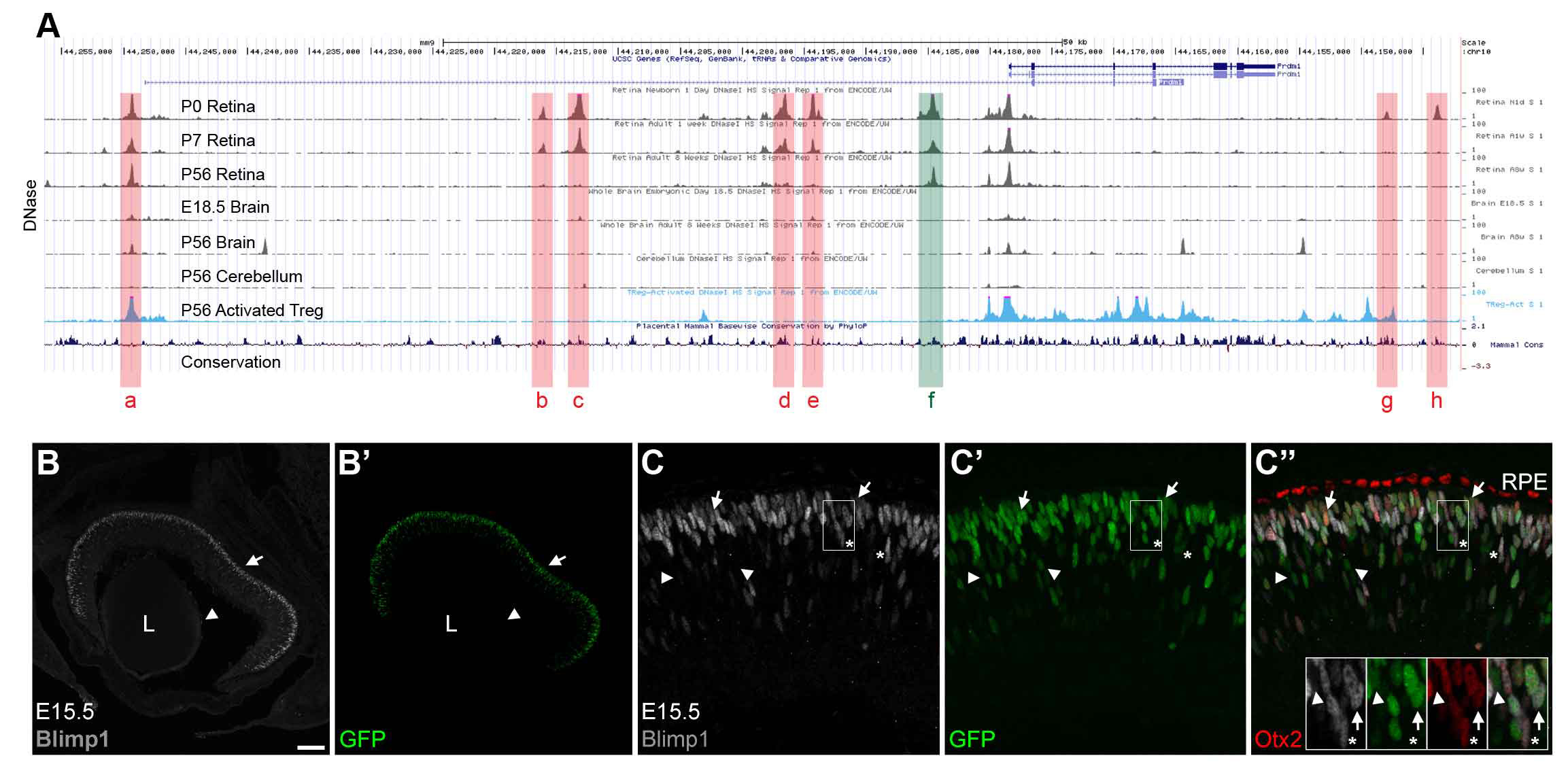
All retinal neurons are formed before we are born. The retina starts out as a population of progenitor cells (similar to stem cells). These progenitors are multipotent, that is they can become any of the seven major cell types of the retina. Over time, these progenitors permanently exit the cell cycle (stop dividing) and differentiate (mature) into neurons and glia. Different cell types are formed at different times. For example cones are formed early and bipolar cells are formed late in development. While there is a trend to when each cell type is formed, there is considerable overlap such that multiple cell types are forming at any given time. How do these progenitors decide which type of cell to become? Development is regulated by genetic programs that dictate a cell’s behavior. Our goal is to understand how these genetic programs control retinal development. This could then be applied to produce cone photoreceptors from stem cells, which may restore vision in patients with diseases like AMD (see CellSight).
Special Thanks to:
- National Institutes of Health
- The Boettcher Foundation
- Department of Defense
- The Glendorn Foundation
- The Lyda Hill Foundation
- The LGA Family Foundation
- The New L Family Fund
- Research to Prevent Blindness, Inc.

 Joe Brzezinski, Ph.D.
Joe Brzezinski, Ph.D. Ko Uoon Park, M.S.
Ko Uoon Park, M.S. Michael Kaufman, M.S.
Michael Kaufman, M.S.

 Noah Goodson, M.S.
Noah Goodson, M.S.