Lab Overview
 Joshua M. Thurman, MD is the Temple Hoyne Buell Professor of Medicine in the Division of Nephrology and Hypertension at the University of Colorado. For more than 25 years, the Thurman laboratory has studied the underlying causes of immune-mediated kidney disease and developed novel diagnostic and therapeutic tools for treating these diseases.
Joshua M. Thurman, MD is the Temple Hoyne Buell Professor of Medicine in the Division of Nephrology and Hypertension at the University of Colorado. For more than 25 years, the Thurman laboratory has studied the underlying causes of immune-mediated kidney disease and developed novel diagnostic and therapeutic tools for treating these diseases.
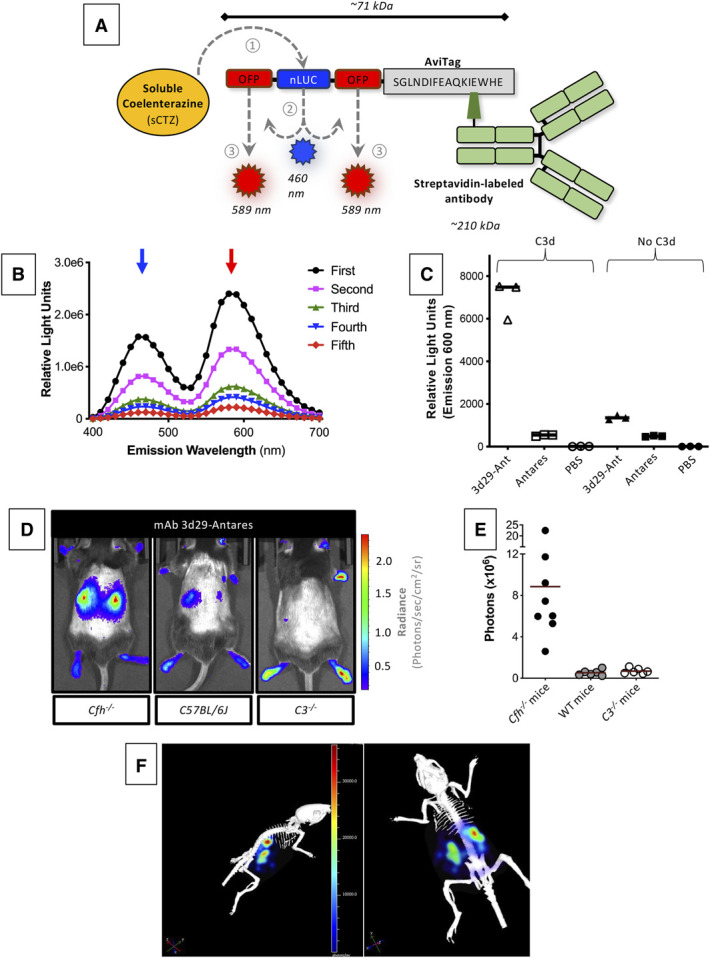
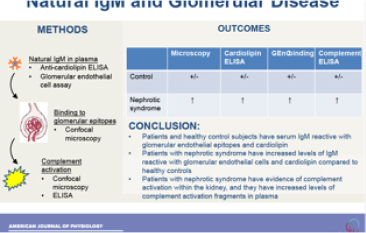
The drugs that are currently available for treating autoimmune disease block the immune system throughout the body, yet inflammation is caused by localized molecular events within affected organs. Our overall goal is to develop new methods for detecting and blocking these events locally. This will allow personalized treatment approaches that are more effective than current therapies, and that have fewer side effects. In pursuit of this goal, we have explored the mechanisms that trigger kidney inflammation after aseptic injuries, such as ischemia/reperfusion or exposure to toxins. We have discovered that injury in diseases not traditionally thought of as autoimmune - such as ischemia/reperfusion - perturbs local complement regulation in the kidney. This work provides a mechanism by which local injury engages the systemic immune system. Based on these findings, we have developed several targeted anti-inflammatory therapeutics, one of which is currently in clinical development. We have also developed novel methods for monitoring tissue inflammation, including magnetic resonance imaging (MRI) and positron emission tomography (PET) based probes that can non-invasively detect complement activation in target organs.