Lab Overview
Charles L. Edelstein, MD is Professor of Medicine in the Division of Nephrology and Hypertension at the University of Colorado. For more than 25 years, the Edelstein laboratory has performed translational research on autosomal dominant polycystic kidney disease (ADPKD) and acute kidney injury (AKI).
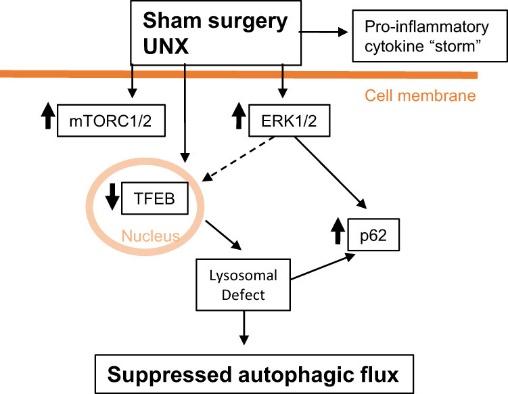
ADPKD is the commonest hereditary kidney disorder. ADPKD leads to chronic kidney disease often requiring dialysis and kidney transplantation. Our ADPKD studies in rats and mice have focused on cell signaling pathways that cause the cells lining the kidney cysts to proliferate, undergo cell death (apoptosis) and undergo mechanisms of cell survival (autophagy) resulting in growth of the cysts. We were the first to describe the activation of caspase-3, the major mediator of apoptosis, in PKD. To determine the role of apoptosis in PKD, we inhibited caspase-3 in rodent models of PKD and found that both genetic and pharmacological methods of caspase and apoptosis inhibition decreased tubular cell proliferation, slowed PKD and improved kidney function. These studies demonstrated the crucial and deleterious role of caspase-3-mediated apoptosis in PKD. As the mammalian target of rapamycin (mTOR) pathway plays an important role in apoptosis, we studied mTOR signaling in PKD. We demonstrated that mTOR inhibition with rapamycin, mTOR kinase inhibitors or an mTOR antisense oligonucleotide (ASO) slows the progression of PKD and improves kidney function in experimental models of PKD. mTOR signaling and apoptosis is integrally linked with a process called autophagy, that helps cells to survive various insults. We published the first report of decreased autophagy in polycystic kidneys. We have developed novel techniques to determine autophagic flux both in vivo in the kidney and in vitro in tubular cells. Cardiac disease is a common cause of death in patients with ADPKD. We have described for the first time that there is activation of AMPK, increased mTORC1/2, suppressed autophagy and cardiac hypertrophy in hearts from PKD mice. Our current studies focus on novel mTOR signaling pathways in both the kidneys and heart in experimental PKD models.
AKI is a common acute kidney disorder that results mainly from systemic sepsis and shock and has a high mortality in patients in the intensive care unit. In 1995 we described the role of the cysteine protease, calpain, in AKI. In the late 1990s a group of cysteine proteases called caspases, that mediated programmed cell death or apoptosis, were discovered. We focused on caspase-1, also known as Interleukin-1 converting enzyme or ICE, that activates the cytokines IL-1 and IL-18. In two papers in J. Clin. Invest. in 2001 and 2002, we described that IL-18 was both a mediator and biomarker of AKI and that the injurious effect of IL-18 in AKI was independent of neutrophils. Recently, we discovered that IL-33, another IL-1 family cytokine that is activated by caspases, is a mediator of cisplatin-induced AKI.
Our mouse studies in 2001 that IL-18 increases in the kidney and urine in AKI were the first suggestion of the use of biomarkers that come from the kidney to diagnose AKI rather than serum creatinine that has been used since 1917. The IL-18 work was taken to the bedside and we received a patent in 2006 for IL-18 as a biomarker of AKI in humans. We demonstrated that IL-18 is an early biomarker of AKI that increases before serum creatinine in patients with delayed graft function and patients post cardiac surgery.
In summary, our laboratory has made original discoveries on the role of the cysteine proteases and cysteine protease-derived cytokines in both PKD and AKI. Both our IL-18 biomarker studies in AKI and our mTOR studies in rodents with PKD have been translated to the bedside.