 pMTM Study
pMTM Study
Optimizing the Clinical Management of Polypharmacy for Children with Medical Complexity (CMC)
Funded by the Agency for Healthcare Research and Quality under Award #: R01 HS028979.
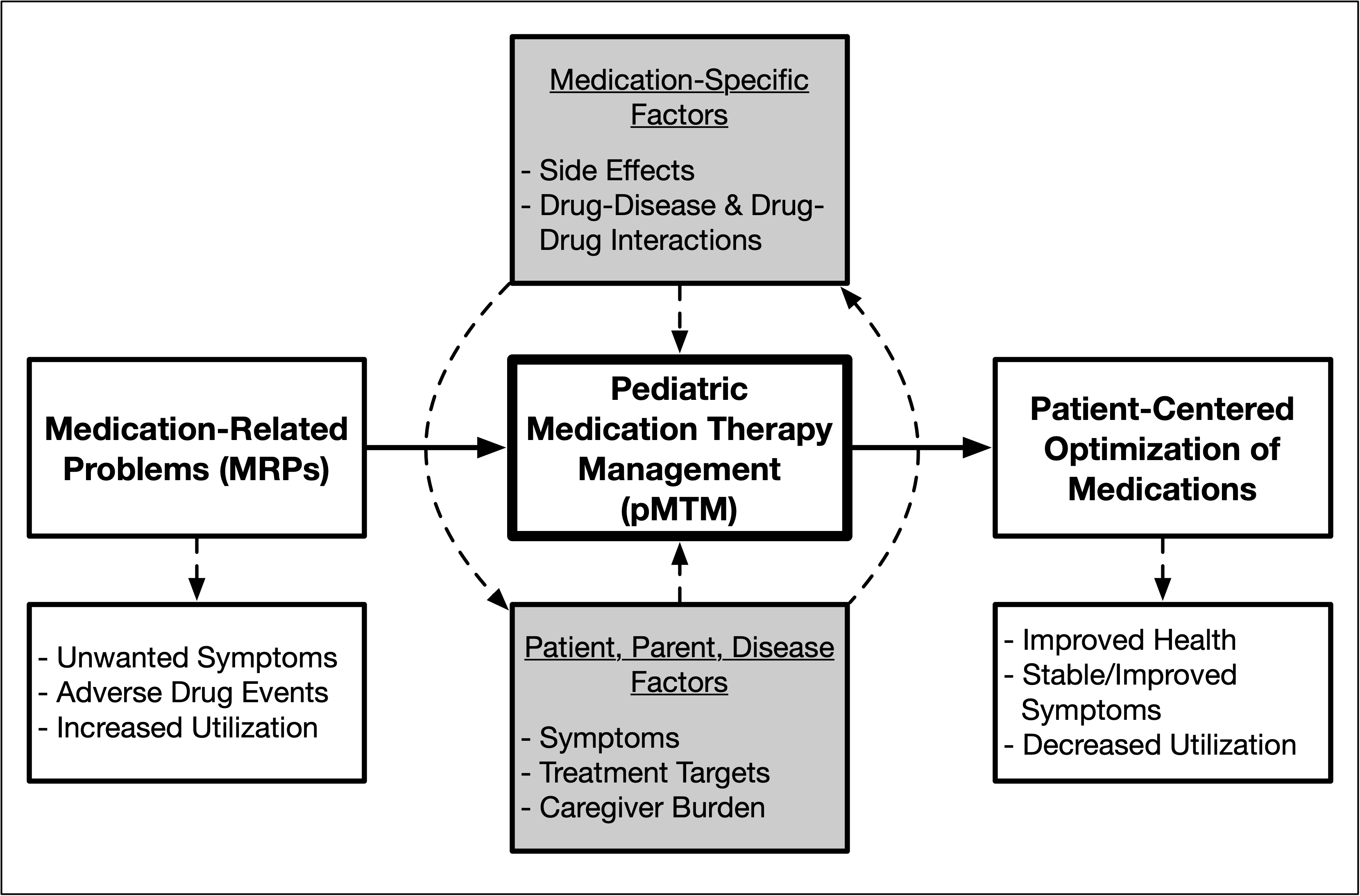
The purpose of this study (ClinicalTrials.gov: #NCT05761847) is to evaluate whether an intervention called Pediatric Medication Therapy Management (pMTM) improves the identification and management of medication-related problems among children with medical complexity and polypharmacy.
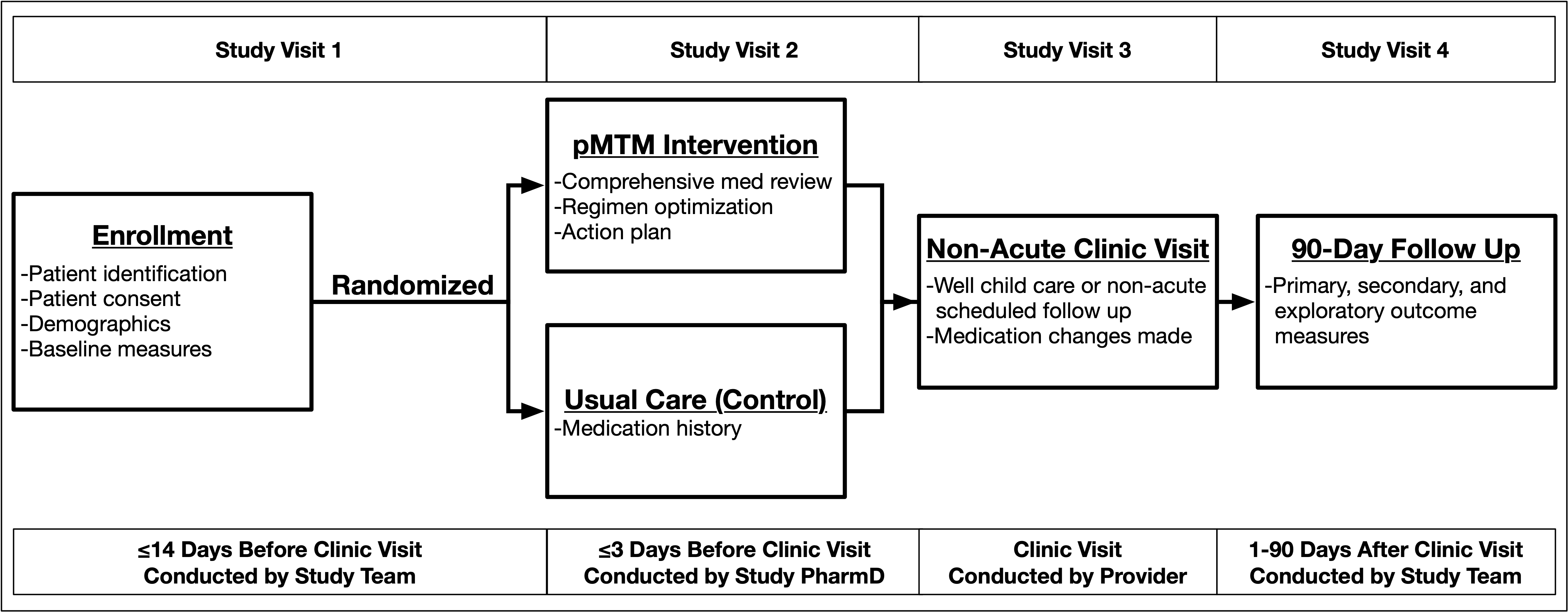
Patients and their parents who meet the study eligibility requirements will be invited to participate in the study. After being informed about the study and potential risks, all patients and their parents giving written informed consent (and assent, when appropriate) will be randomized in a 1:1 ratio to usual care or to the Pediatric Medication Therapy Management (pMTM) intervention.
| Participant Group/Arm | Intervention/Treatment |
Intervention: Pediatric Medication Therapy Management (pMTM) | The intervention group will receive the Pediatric Medication Therapy Management (pMTM) study intervention before a routine clinical visit. The pMTM intervention is comprised of 3 activities: 1) comprehensive review of the medication regimen; 2) optimization of the medication regimen; and 3) creation of the medication action plan. |
No Intervention: Usual Care | The usual care group will receive standard-of-care medication management practices delivered during the course of a routine clinical visit. |
| Outcome Measure | Primary or Secondary | Measure Description | Time Frame |
| Medication-Related Problem (MRP) Count | Primary | A MRP is a clearly defined event involving medication therapy that interferes with an optimum outcome for a specific patient, including: inappropriate or unnecessary therapy; suboptimal therapy; undertreated symptom; adverse drug event; major drug-drug interaction; duplication of therapy; or, unclear prescription instruction. | 90 Days |
| Change in Parent-Reported Outcomes of Symptoms (PRO-Sx) Global Symptom Score | Secondary | The PRO-Sx instrument assesses 28 physical and psychological symptoms over the past week. The study instrument is designed to be completed by a full-proxy parent and contains 28 symptom items, each with 4-point scores for domains of frequency, severity, and extent of bother. Based on these components, a global symptom score and individual symptom scores can be calculated (0-100 scale, with 100 being the worst). Change equals the 90-day score minus the baseline score. | Baseline and 90 Days |
| Acute Healthcare Visit Count | Secondary | Unplanned acute healthcare visits include: ambulatory sick visits; emergency room visits; and, inpatient hospitalizations. | 90 Days |
Please contact us at [email protected]