
Research and Innovation—July, 2022
SELECTED RESEARCH HIGHLIGHTS |
FDA Expands Breyanzi Approval for Relapsed or Refractory Large B-Cell Lymphoma
The FDA approved lisocabtagene maraleucel for treatment of adults with relapsed or refractory large B-cell lymphoma after one prior therapy. The agency based the new indications on results of the phase 3 TRANSFORM study, led by Manali Kamdar, MD, associate professor of medicine, Division of Hematology, which included adults with large B-cell lymphoma that was primary refractory to or relapsed within 12 months of front-line therapy.
Tall Height Impacts Risk of Multiple Diseases
Sridharan Raghavan, MD, assistant professor of medicine, Division of Hospital Medicine, Rocky Mountain Regional VA Center, is the first author of the study, “A multi-population phenome-wide association study of genetically-predicted height in the Million Veteran Program.” The study examined genetic and health data from 250,000 veterans from the VA Million Veteran Program, using genetic tools to identify the relationship between height and the development of diseases. Previous studies aimed at linking tall height to diseases examined up to 50 genetic traits; now, researchers looked at more than 1,000 traits and conditions, making it the largest study of the relationship of height to health conditions to date.

Jose R. Castillo-Mancilla, MD, and Team Receive Second R01
Jose R. Castillo-Mancilla, MD, associate professor of medicine, Division of Infectious Diseases, and colleagues received their second R01 for their research through 2026 on “New Pharmacologic Measures of ART Adherence and Exposure: Pathway to Clinical Implementation.” This is a new line of research on the pharmacology of long acting antiretrovirals.

Fan Zhang, PhD, Publishes Science Translational Medicine Paper
Fan Zhang, PhD, assistant professor of medicine, Division of Rheumatology, is the first co-author of a paper in Science Translational Medicine, “Granzyme K+ CD8 T cells form a core population in inflamed human tissue”.
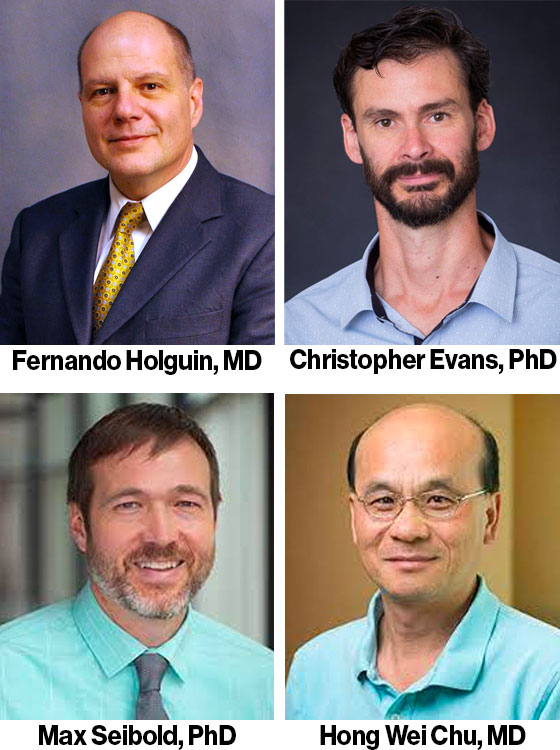
Pulmonary Team Receives CU ASPIRE Program Award
Fernando Holguin, MD; Chris Evans, PhD; Max Seibold, PhD; Hong Wei Chu, MD, Division of Pulmonary Sciences and Critical Care Medicine, received the CU ASPIRE Program award for programmatic research. The CU ASPIRE Program is designed to facilitate collaborative research groups working on unmet needs in basic science or clinical medicine that can only be addressed by a team of investigators.

Erin Schenk, MD, PhD Receives Translational Scholar Award
Erin Schenk, MD, PhD, assistant professor of medicine, Division of Medical Oncology, is the recipient of the School of Medicine’s 2022 Translational Research Scholars Program award. The goal of the program aims to foster translational research among the School’s early career faculty.

Hospital Medicine Team Publishes JHM Article
Marisha Burden, MD; Hemali Patel, MD; Mark Kissler, MD; Elizabeth Harry, MD; Angela Keniston, MSPH, Division of Hospital Medicine, published “Measuring and driving hospitalist value: Expanding beyond wRVUs” in the Journal of Hospital Medicine.

Bryan Haugen, MD, Receives Core Grant Award
Bryan Haugen, MD, head of the Division of Endocrinology, Metabolism and Diabetes, received the Parse Biosciences Evercode Whole Transcriptome Kit for his innovative work to elucidate kinase inhibitor response in MAPK-pathway driven thyroid cancers.
PROGRAMS AND FUNDING OPPORTUNITIES |
Multidisciplinary Research Training in Pulmonary Disease Training Grant (T32)
 Post-doctoral
training positions are available on the Multidisciplinary Research Training in Pulmonary Disease Training Grant (T32) for academic year 2022–23. If
you are working with a post-doctoral PhD or research fellow MD who qualifies for training support and you would like to be considered for an appointment, please click here to learn more.
Post-doctoral
training positions are available on the Multidisciplinary Research Training in Pulmonary Disease Training Grant (T32) for academic year 2022–23. If
you are working with a post-doctoral PhD or research fellow MD who qualifies for training support and you would like to be considered for an appointment, please click here to learn more.
Application materials should be sent to Cheryl Loudd by July 18, 2022. The Research Oversight Committee (ROC) of the Pulmonary T32 program will make a decision at the end of July. Learn more.
 Call
for Applications: University of Colorado Interdisciplinary Joint Biology Pilot Program
Call
for Applications: University of Colorado Interdisciplinary Joint Biology Pilot Program
The 2022–23 grant cycle will fund new initiatives for investigators who are pursuing joint-biology related research that should lead to NIH K/R type funding or other extramural support (e.g. foundations, industry, Department of Defense, etc.). All
application materials for pilot projects are due October 1, 2022 by 5pm MST. Click here to learn more or see the list of
past grant recipients.
 CU
Innovations Startup ToolBox
CU
Innovations Startup ToolBox
Attention CU Inventors! Does your research have the potential to transform patient lives? CU Innovations Startup Toolbox can help with your commercialization needs. Apply for a microgrant here.
Ludeman Family Center for Women’s Health Research Opportunities
- National Research Conference on Women’s Health and Sex Differences, Oct 12-14, 2022, Colorado Springs. Learn more.
- The Ludeman Family Center for Women’s Health Research is offering a $2,500 award for faculty doing outstanding research in women’s heart health. Eligible candidates will be evaluated on criteria appropriate to measure progress in research. Learn more.
Updated NIH Data Management and Sharing Policy
Effective Jan 25, 2023, the NIH will implement an updated Data Management and Sharing Policy, which applies to all NIH-supported research that results in generation of scientific data. The updated policy will require a detailed
Data Management and Sharing plan to be submitted with grant applications, and this plan will apply to any data resulting from the funded project.
Please direct any questions to Jennifer Kemp, PhD, director of the DOM Research Office.
New HHMI Program Pledges $1.5 Billion for Outstanding Early Career Faculty Committed to Diversity, Equity, and Inclusion
The Freeman Hrabowski Scholars Program, through the Howard Hughes Medical Institute, is now accepting applications from outstanding basic biomedical researchers, including physician-scientists, who are strongly committed to advancing diversity, equity, and inclusion in science. Each Freeman Hrabowski Scholar will receive up to $8.6 million over 10 years, including full salary, benefits, a research budget and scientific equipment. In addition, Scholars will participate in professional development to advance their leadership and mentorship skills. Learn more.
WEBINARS, EVENTS AND CONFERENCES |
SPARK|Reach Webinar Series
SPARK|Reach Webinar Series—July 13, 4-6pm: Getting the Information You Need: Customer Discovery
, presented by Cathy Bodine, PhD, CCC-SLP. Register now.
See the full schedule of webinars and register on the SPARK events page.



