Methodology
The iC42 Clinical Toxicology Laboratory uses Liquid Chromatography-Tandem Mass Spectrometry and an extensively developed drug assay to produce highly accurate results for clinicians around Colorado. Laboratory technicians can detect over a hundred drug analytes or metabolites that indicate drug use with a single assay and quantify results with extensively validated data analysis methods.
Utilizing High-Performance Liquid Chromatography-coupled Mass Spectrometry (LC-MS/MS) allows for the analysis of urine and blood samples for a high number of analytes to inform clinicians who can then provide the correct treatments.
As a clinical laboratory, there are many aspects that differ from a research lab. The accuracy of the data analysis is highly important. The assay used has been extensively developed and validated, and the instruments used in the laboratory are able to produce highly accurate and precise results.
Additionally, the ability to communicate results from analyses with clients and work efficiently is extremely important. With the high number of samples analyzed and analytes being tested, there are a lot of complications to consider, minimizing the chance of systematic and random errors.
The laboratory services performed include comprehensive urine testing for 136 drug analytes or metabolites in a single assay using highly accurate and precise LC-MS/MS analysis. While most clinical laboratories use immunoassay for detection of drug use in patients, the addition of confirmatory analysis such as LC-MS/MS for positive immunoassay hits is mandatory.
The important aspects of HPLC and mass spectrometry, and how to resolve common issues will be outlined. Moreover, it is critical to identify urine samples that have potentially been adulterated. Thus, the systems used to quantify creatinine, pH, general oxidants, and specific gravity to monitor adulteration will be explained.
A full accumulation of the assay run on urine samples can be found under Laboratory Services and Resources. When considering the interpretation of the results of toxicology testing, each drug analyte or metabolite divided into its drug class has specific aspects to remember. It is important to outline the cutoffs and confirmational values used for each drug, as well as any complications that come with testing for each analyte.
The importance of providing accurate results in a clinical toxicology laboratory should not be downplayed. The clinicians who provide care and treatments to patients depend on highly precise results from an assay that shows concentrations of each analyte or metabolite that was present when the sample was collected.
The detection of an analyte that was not present or the failure to detect something that was taken could have negative implications on the patients’ treatments or legal implications, especially if children are involved.
The clinical work performed at the iC42 laboratory is for the benefit of clinicians who require accurate information about their patients, and the resources they may require regarding the assay or patient results can be found here.
Immunosassay LC-MS/MS
The use of liquid chromatography as a confirmatory spectroscopic method along with preceding immunoassay analysis for drug analyte detection has been extensively researched in forensic and clinical toxicology laboratories over recent years. Immunoassays alone allow for the detection of drug analytes above a certain cutoff concentration.
| Test Analyte | Metabolite | Immunoassay Test Cutoff (ng/mL) | Confirmatory Test Cutoff (ng/mL) | Detection Time |
| Cocaine | Benzoylecgonine | 150 | 100 | 2-4 days |
| Ecstasy | MDMA, MDA | 500 | 250 | 2-4 days |
| Heroin | 6-Acetylmorphine | 10 | 10 | 48 hours |
| Marijuana | THCA | 50 | 15 | 3 days (single use) 10-15 days (daily use) |
| Meth | Amphetamine, Methamphetamine | 500 | 250 | 48 hours |
| Opiates | Codeine, Morphine | 2,000 | 2,000 | 48 hours |
| OxyContinÒ | Oxycodone, Oxymorphone | 100 | 100 | 2-4 days |
| PCP | Phencyclidine | 25 | 25 | 8 days |
| VicodinÒ / DilaudidÒ | Hydrocodone, Hydromorphone | 300 | 100 | 2-4 days |
SAMHSA cutoff levels from 2017 for common drugs of abuse, and the window of time where the analyte is detectable.5, 6
The Substance Abuse and Mental Health Services Administration has published cutoff levels and detection time frames for immunoassay analysis that minimizes false negative and positive results but are not appropriate for highly sensitive clinical analysis.1 Values below this cutoff will produce a negative result for the analyte, meaning drugs that were taken by patients may not be detected resulting in a false negative.
Additionally, due to the way molecules bind to antibodies in immunoassays, it often times fails to differentiate between structurally similar compounds, and there is a possibility for false positive results.2 Some common false positives that have been noted from immunoassays are poppy seeds testing positive for opioid use, NSAIDS such as ibuprofen resulting in a false diagnosis of barbiturate use, and morphine or codeine resulting in a false diagnosis of buprenorphine use.2
The advantage of using a structure-confirming method along with immunoassay testing includes minimizing false negative or positive results. While specific immunoassays that test for one drug or drug class are accurate, the ability for chromatographic methods to test for any number of analytes at once makes it an important method for analyzing samples that have many unknowns.
The SAMHSA provides additional confirmatory levels that may be used for urine drug screens with alternate technologies such as LC-MS/MS. This additional analysis allows for quantitative results that confirm or contradict the immunoassay results.
The use of LC-MS/MS alone has several benefits and some down-sides. There is a greater window of time after use of the drug that detection is possible with LC-MS/MS, due to its higher sensitivity compared to common immunoassays. The ability to detect trace amounts, below SAMHSA cutoffs, and separate similar analyte structures greatly reduces the amount of false negative results. In a recent study comparing LC-MS/MS performance with the immunoassay EMIT in the detection of sirolimus, it was found that the chromatographic method had greater reproducibility and more accurate concentrations.3
Another study comparing LC-MS/MS analysis with two immunoassays for the detection of benzodiazepines in urine showed that, while each were deemed successful screening tools, they also produced false positive and false negative results.4 Chromatographic methods had a much lower probability of producing false positive and false negative results and would be the preferred method for quantitative benzodiazepine drug testing. The downside of using LC-MS/MS alone is that analytes that are not included in the assay or method will not be detected. Additionally, there are substantial costs and maintenance required to ensure the results are accurate.
The assay used in the iC42 Clinical Toxicology Laboratory has specific challenges due to the high number of analytes that are included. The processes put in place including quality control samples in each analytical batch as well as weekly, monthly, and annual quality control tracking, quality assurance procedures, and validations ensure the consistent quality of results. This means the results can be considered accurate and containing substantially fewer false positives or negatives that are common with immunoassay analysis.
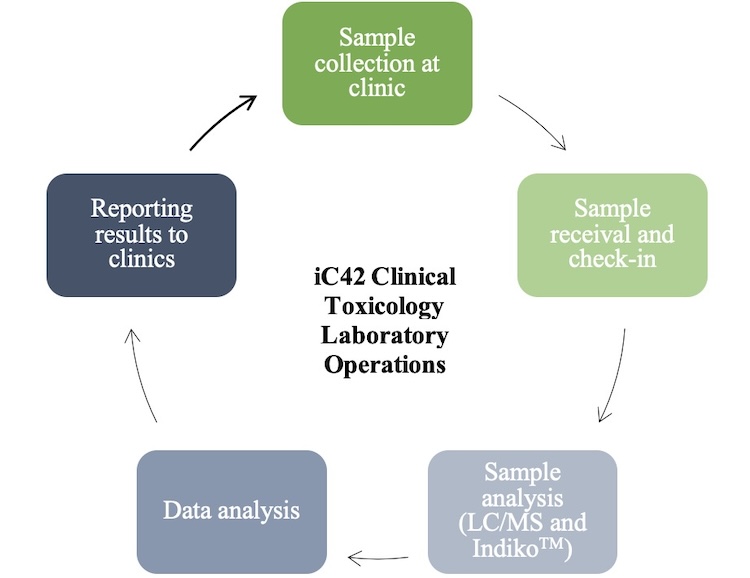
Laboratory Workflow
The workflow of the laboratory allows for quick analysis and samples and reporting of results, all within 24 to 48 hours. After collection of samples with the clinic, the samples are received and checked into the laboratory information management system (LIMS). Chain of custodies are organized and filed, and each sample is checked into the online system to ensure the results will be reported for the correct patient and clinic.