Benjamin Scholl
Assistant Professor
/image-180x180.jpg?sfvrsn=6e15d6bb_0)
RC1 North Tower, 7th floor
Department of Physiology and Biophysics
University of Colorado School of Medicine, Denver, CO 19104
Tel (971) 645-5409
E-mail: benjamin.scholl@cuanschutz.edu
External Website : https://benjaminscholl.com/
Graduate Program Affiliations:
Neuroscience Graduate ProgramBioengineering Graduate Program
Scholl_cv_current
(pdf)
89 KB
Shared Content Block:
Physiology & Biophysics Styles -- Borderless table for directories
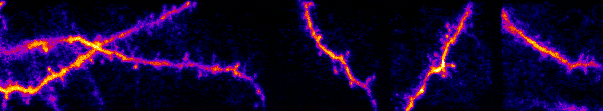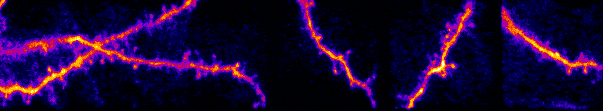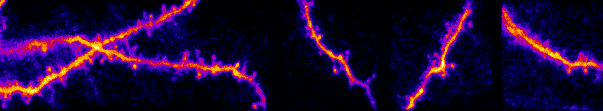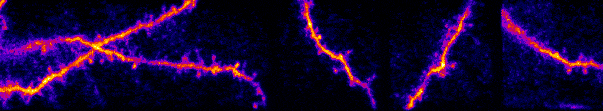
I am interested in mechanisms of cortical sensory processing: How do neurons transform information from the world and into electrical activity used to mediate perception and behavior. Previously I worked in the auditory system, but I now focus on the
visual system. I have studied the primary visual cortex in a number of mammalian species and am a proponent of the power of comparative analyses.
My lab studies synaptic networks. Each neuron receives a wide collection of inputs. These
inputs can drive, suppress, or subtly modulate their target. The suite of operations neurons can perform are defined by these inputs and their dynamics. Outside network dynamics, the only way a neuron can dramatically change its operational capacity
is through plasticity (e.g. learning, development) of these inputs. Thus, to understand how individual neurons and circuits transform information, we must build a fundamental understanding of synaptic networks. I believe this knowledge will extend
beyond fundamentals and lead to novel insights into circuits in neurological and developmental disorders.
Our work uses a variety of techniques to study sensory processing within single cells and across large-scale populations in vivo.
Historically this has been with electrophysiology (intracellular and extracellular recordings) and multiphoton calcium imaging. We have expanded to two-photon optogenetics, functional connectomics with electron microscopy, novel viral constructs,
and gene editing to disrupt naturally expressed proteins and receptors.
Project 1:
Visualizing synapses in action during cortical circuit function
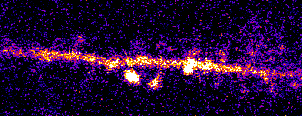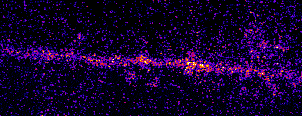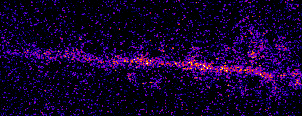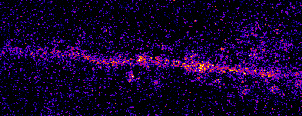
Single neurons are driven by huge populations of synapses. In the visual system, single neurons are highly selective for
features in the world (often in an invariant manner). In carnivores and primates, a tremendous amount of visual selectivity emerges in the primary visual cortex. This project aims to understand how selectivity emerges in single neurons; how synaptic
inputs are transformed into somatic outputs (i.e. spikes). This project uses in vivo multiphoton calcium imaging of synaptic networks on single pyramidal cortical neurons, taking advantage of dendritic spines which are major site of excitatory input
innervation. Understanding input/output transformations in single neurons in the visual system will provide much needed insight into the nature of neural computation.
Project 2:
Probing the behavior of cortical circuits in action
Each neuron’s population of presynaptic partners determines how incoming information is processed. A majority of synaptic inputs originate from local networks through horizontal (recurrent) connections. Theoretical models of the visual system suggest
these inputs perform a fundamental function in cortical circuits: selective modulation. Specifically, selective amplification or attenuation in a manner dependent on the strength and statistics of incoming sensory information. But this process has
not been elucidated at the synaptic level, and the mechanisms proposed by stem from studies of rodents and have not been tested in different mammalian systems. This project aims to map presynaptic excitatory and inhibitory cells of single layer 2/3
neurons and dissect how they act to selectively modulate the behavior of neural circuits in a carnivore. This project utilizes a combination of in vivo multiphoton optogenetic neural control, multiphoton calcium imaging, and intracellular electrophysiology.
-(1).gif?sfvrsn=f025d3bb_1/
optostim_project_2-(1)-(1)
.gif)
Project 3:
Visual development of natural behavior (hunting ferrets) and neural dynamics
Neural development is generally considered a feed- forward process: experience shapes neural activity, thereby influencing the structure, gene expression, and function of brain circuits. Missing from this picture is the dynamic nature of experience itself, which is shaped by developing brain circuits, and how natural behaviors concurrently emerge with maturing visual circuits. To address this challenge, we propose a return-to-first-principles approach using the ferret model system. We characterize the developmental trajectory of natural visually-guided behaviors and map the concurrent maturation of neuronal circuits. We are using modern methods and computational tools to track the natural development of visually-guided behaviors, kinematics, and eye-movements in ferrets. We will incorporate head-fixed and head-mounted in vivo two-photon (2P) microscopy to map the structural and functional dynamics of neural networks at synaptic and cellular scales. This project will produce a novel platform for studying the intersection between dynamic experience, changes in statistics of the outside world, constraints of neural circuits, and the development of natural behaviors.
/project-3.png?sfvrsn=8f6ed3bb_0)
Project 4:
Single-cell CRISPR/Cas9 manipulations to study molecular mechanisms and synaptic pathology in cortical spines, cells, and circuits
Synaptic pathology is a prominent feature of psychiatric illness and many neurological disorders. For example, synaptic dysfunction is presumed to be an underlying cause of Autism Spectrum Disorder, as genomic studies have identified risk genes regulating synaptic structure and physiology, and NMDA receptor dysfunction is highly implicated in the emergence of schizophrenia, based on numerous studies from mouse to primates and humans. One gene strongly associated with ASD and other CNS disorders is Phosphatase and tensin homolog located on chromosome 10 (Pten). But while global mouse models with dysfunctional Pten exhibit synaptic pathology and mimic ASD symptoms, it is unknown how synaptic integration, organization, and function are impacted within individual neurons. This project aims to understand how Pten signaling shapes synaptic integration and functional architecture in single neurons. This collaborative project uses CRISPR/Cas9 edits in single neurons in combination with in vivo synaptic calcium imaging measurements.
.png?sfvrsn=b6dd3bb_1/
fig2_project_4-(2).png)
/pic_lk-(1).jpg?sfvrsn=96ced3bb_1/
pic_lk-(1)
.jpg) |
Laura Koek Postdoc |
/240417penn_1113-(1).jpg?sfvrsn=99ced3bb_1/
240417penn_1113-(1)
.jpg)
|
Joe Barreto Professional Research Assistant |
/silhouette-100x125.png?sfvrsn=631d62b9_4) |
Greg Bond Professional Research Assistant |
Alfred P. Sloan Research Fellow (2023)
Brain Research Foundation Seed Grant Award (2023)
IDDRC Program Development Award (2023)
University Research Foundation Award (2023)
Whitehall Foundation Award (2022)
VRC Pilot Award (2021)
McCabe Fund Award (2021)
Allison Doupe Fellowship Award (2019)
MPFI Scientific Achievement (2018)
Neurizons Young Investigator (2016)
NSF Graduate Research Fellowship Honors (2012)
United States Representative to Nobel Laureate meeting for Physiology and Medicine (2011)
Thomas C, Ryan M, McNabb MC, Kamasawa N, and Scholl B (2024) Astrocyte coverage of excitatory synapse correlates to measures of synaptic structure and function in primary visual cortex. bioRxiv. https://www.biorxiv.org/content/10.1101/2023.12.01.569664v1
Thomas C, Ryan M, Kamasawa N, and Scholl B (2023) Postsynaptic mitochondria are positioned to support functional diversity of dendritic spines. eLife. https://elifesciences.org/reviewed-preprints/89682
Yates JL and Scholl B (2022) Unraveling functional diversity of cortical synaptic architecture through the lens of population coding. Frontiers in Synaptic Neuroscience. https://doi.org/10.3389/fnsyn.2022.888214
Scholl B*, Tepohl C*, Thomas C, Ryan M, Kamasawa N and Fitzpatrick D (2022) A Binocular synaptic network supports interocular response alignment in visual cortical neurons. Neuron. https://doi.org/10.1016/j.neuron.2022.01.023
Scholl B*, Thomas C*, Ryan M, Kamasawa N and Fitzpatrick D (2021) Cortical neuron response selectivity derives from strength in numbers of synapses. Nature. https://www.nature.com/articles/s41586-020-03044-3
Wilson DE*, Scholl B* and Fitzpatrick D (2018) Differential tuning of excitation and inhibition underlies direction selectivity in ferret visual cortex. Nature.https://www.nature.com/articles/s41586-018-0354-1
All people deserve to be treated equally, with dignity and respect. Our lab welcomes and encourages individuals from all backgrounds. We are committed to maintaining and promoting a diverse, inclusive, and supportive environment. Together, we strive for scientific excellence while learning and honing a variety of skills in the process.